Please set your exam date
Maternal Risk Factors
Study Questions
Practice Exercise 1
A 16-year-old primigravida presents for her first prenatal visit. Which of the following risks is specifically heightened due to her adolescent age?
Explanation
Adolescent pregnancy is associated with unique risks due to incomplete pelvic growth, hormonal changes, and limited nutritional reserves. Girls <18 years often have smaller pelvic diameters, predisposing them to cephalopelvic disproportion. Hemoglobin should normally be 12–16 g/dL in females, but iron deficiency anemia is common in adolescents. Normal maternal blood pressure is <120/80 mmHg; adolescents are less likely to have chronic hypertension compared to older women. Chromosomal abnormalities risk is strongly linked to advanced maternal age >35 years, not adolescence.
Rationale for correct answer
3. Cephalopelvic disproportion is more likely in adolescents due to incomplete pelvic maturation. A smaller bony pelvis increases the chance of obstructed labor. The question stem highlights her young age (16 years), which is the main factor raising the risk for CPD.
Rationale for incorrect answers
1. Gestational diabetes mellitus occurs more frequently in women >25 years, especially with obesity or family history. Adolescents generally have higher insulin sensitivity, and the pathophysiology does not favor increased risk in this group.
2. Chromosomal abnormalities, such as trisomy 21, are linked to advanced maternal age because of meiotic nondisjunction. The risk at age 16 is extremely low compared to women >35 years.
4. Placenta previa incidence increases with maternal age, multiparity, and uterine scarring from surgeries. A primigravida adolescent without prior uterine surgery has no significant predisposition to placenta previa.
Take home points
- Adolescent pregnancy increases risk of cephalopelvic disproportion due to incomplete pelvic growth.
- Gestational diabetes mellitus is more common in older pregnant women.
- Chromosomal abnormalities are strongly associated with advanced maternal age.
- Placenta previa risk rises with prior uterine surgery, advanced maternal age, and multiparity, not adolescence.
Which of the following maternal age groups is associated with an increased risk of chromosomal abnormalities such as Down syndrome?
Explanation
Chromosomal abnormalities result from errors in meiotic division, particularly nondisjunction, leading to conditions such as trisomy 21 (Down syndrome). The risk of nondisjunction increases with advancing maternal age, especially ≥35 years. Normal karyotype is 46 chromosomes. The baseline risk for trisomy 21 at age 25 is approximately 1 in 1,250, while at age 35 it rises to about 1 in 350.
Rationale for correct answer
4. Maternal age ≥35 years is strongly associated with chromosomal nondisjunction events during oogenesis. This significantly increases the risk of Down syndrome and other trisomies. The question directly asks for the age group linked to increased chromosomal abnormality risk, making this option correct.
Rationale for incorrect answers
1. The age group 18–24 years carries a very low risk for chromosomal abnormalities, with Down syndrome occurring in about 1 in 1,400 live births at age 20. Nondisjunction errors are uncommon in this group.
2. Women aged 25–30 years have only a slightly increased risk compared to teenagers, with trisomy 21 risk around 1 in 1,000. This is not considered a high-risk age group.
3. Ages 31–34 years show a gradual increase in risk, with trisomy 21 risk at age 32 being about 1 in 900. However, this group is not defined as high risk until ≥35 years.
Take home points
- Risk of chromosomal abnormalities increases significantly after maternal age 35.
- Nondisjunction is the primary mechanism leading to trisomies such as Down syndrome.
- Younger maternal age groups have a very low baseline risk.
- Counseling and screening are emphasized for women ≥35 years.
Which of the following are risks associated with adolescent pregnancy? Select all that apply.
Explanation
Adolescent pregnancy carries medical and psychosocial risks due to biologic immaturity, inadequate prenatal care, and poor nutritional status. Young mothers <18 years are more likely to experience preterm birth (<37 weeks) and deliver infants with low birth weight (<2,500 g). Hemoglobin is often <12 g/dL, reflecting iron deficiency anemia. Adolescent mothers are also more vulnerable to intimate partner violence, with reported prevalence rates up to 20–30%. Normal pregnancy blood pressure is <120/80 mmHg, and risk of preeclampsia is not decreased in adolescence. Cesarean delivery may be required due to cephalopelvic disproportion.
Rationale for correct answer
1. Preterm birth is more common in adolescents because of biologic immaturity and inadequate cervical competence, as well as poor access to prenatal care. This raises risk for neonatal morbidity and mortality.
3. Low birth weight is frequent due to maternal malnutrition, iron deficiency, and increased risk of intrauterine growth restriction. Infants <2,500 g are more likely in adolescent pregnancies.
5. Intimate partner violence risk is elevated in adolescents, related to power imbalances, dependency, and limited social support. This contributes to adverse maternal and fetal outcomes.
Rationale for incorrect answers
2. The risk of preeclampsia is not decreased in adolescents; in fact, it may be increased, especially in first pregnancies due to immunologic factors. A blood pressure ≥140/90 mmHg is diagnostic for hypertensive disorders in pregnancy.
4. Adolescents do not have reduced likelihood of cesarean delivery. In fact, rates may be higher due to cephalopelvic disproportion from incomplete pelvic growth, leading to obstructed labor.
Take home points
- Adolescent pregnancy increases risk of preterm birth and low birth weight.
- Hypertensive disorders of pregnancy are not less common in adolescents.
- Cesarean delivery may be more frequent due to cephalopelvic disproportion.
- Psychosocial risks such as intimate partner violence are more prevalent in adolescents.
Which of the following are complications associated with advanced maternal age? Select all that apply.
Explanation
Advanced maternal age (≥35 years) is linked to obstetric and fetal risks due to oocyte aging, impaired glucose tolerance, and increased placental dysfunction. Risk of trisomy 21 rises from 1 in 350 at age 35 to 1 in 100 at age 40. Normal fasting plasma glucose is <100 mg/dL, but gestational diabetes mellitus is more common with maternal age >35 due to reduced insulin sensitivity. Placenta previa incidence is higher because of age-related endometrial changes. Stillbirth and preterm birth risks increase, not decrease, in advanced maternal age pregnancies.
Rationale for correct answers
1. Gestational diabetes mellitus risk increases with maternal age due to reduced insulin sensitivity and higher rates of obesity. This is strongly associated with advanced maternal age.
2. Down syndrome risk increases sharply after 35 years because of meiotic nondisjunction. Oocyte aging leads to chromosomal errors.
4. Placenta previa is more frequent in women ≥35 years due to endometrial scarring and impaired placental implantation. Advanced maternal age is a recognized risk factor.
Rationale for incorrect answers
3. Stillbirth risk is not reduced with age. In fact, women ≥35 years have higher rates of stillbirth, likely related to placental insufficiency and medical comorbidities.
5. Preterm birth is not lower in advanced maternal age. The risk is increased, partly due to obstetric complications such as hypertension, diabetes, and placenta previa.
Take home points
- Advanced maternal age ≥35 years increases risk of gestational diabetes.
- Chromosomal abnormalities, especially trisomy 21, are strongly age-related.
- Placenta previa incidence rises with older maternal age.
- Both stillbirth and preterm birth risks increase with advanced maternal age.
Practice Exercise 2
A nurse is assessing a pregnant patient with a history of diabetes mellitus. What is a potential fetal complication associated with poorly controlled maternal diabetes?
Explanation
Maternal diabetes mellitus affects pregnancy due to maternal hyperglycemia, altered insulin metabolism, and abnormal placental function. Poor glycemic control leads to excess glucose crossing the placenta, causing fetal hyperinsulinemia and accelerated growth. Normal fasting plasma glucose is <100 mg/dL, and hemoglobin A1C should remain <6.5% during pregnancy. Complications include macrosomia, congenital malformations, neonatal hypoglycemia, and stillbirth.
Rationale for correct answer
2. Macrosomia occurs when maternal hyperglycemia causes excess transplacental glucose transfer. The fetus responds with hyperinsulinemia, which acts as a growth hormone, leading to birth weight >4,000 g. Poorly controlled diabetes is the direct cause in this scenario.
Rationale for incorrect answers
1. Microcephaly is not a classic complication of maternal diabetes. It is more commonly linked to congenital infections such as Zika virus, rubella, or cytomegalovirus, rather than hyperglycemia.
3. Spina bifida results from neural tube defects associated with folate deficiency, not from maternal diabetes. Normal maternal folate level should be >4 ng/mL to prevent such defects.
4. Low birth weight (<2,500 g) is usually associated with maternal hypertension, intrauterine growth restriction, or placental insufficiency. In diabetes, the opposite effect (excess growth) is more common due to increased nutrient availability.
Take home points
- Poorly controlled maternal diabetes leads to fetal macrosomia.
- Macrosomia is caused by fetal hyperinsulinemia from maternal hyperglycemia.
- Neural tube defects and microcephaly are not direct outcomes of diabetes.
- Low birth weight is linked to placental insufficiency, not poorly controlled diabetes.
A patient with a history of chronic hypertension is pregnant. Which of the following complications is she at an increased risk for?
Explanation
Chronic hypertension in pregnancy is defined as blood pressure ≥140/90 mmHg present before conception or before 20 weeks of gestation. It leads to impaired uteroplacental perfusion, abnormal vascular remodeling, and increased placental ischemia. Normal blood pressure is <120/80 mmHg, and mean arterial pressure should be 70–105 mmHg. This condition predisposes to placental abruption, intrauterine growth restriction, preeclampsia, and stillbirth.
Rationale for correct answer
3. Placental abruption is strongly linked to chronic hypertension because long-standing elevated pressure damages spiral arteries, reducing placental attachment stability. Ischemic injury and vascular rupture increase risk of premature placental separation, leading to bleeding and fetal compromise.
Rationale for incorrect answers
1. Neonatal hypoglycemia is more commonly associated with infants of diabetic mothers due to fetal hyperinsulinemia in response to maternal hyperglycemia. It is not a direct complication of chronic hypertension.
2. Macrosomia, defined as fetal weight >4,000 g, is linked to maternal diabetes mellitus due to excess transplacental glucose. In chronic hypertension, placental insufficiency more often causes restricted growth rather than overgrowth.
4. Congenital anomalies are not specifically associated with maternal chronic hypertension. They are more related to teratogenic exposures, poorly controlled diabetes, and genetic causes. Hypertension mainly causes placental complications rather than structural defects.
Take home points
- Chronic hypertension in pregnancy increases risk of placental abruption.
- Placental abruption results from ischemic vascular damage and poor placental attachment.
- Macrosomia and neonatal hypoglycemia are associated with maternal diabetes, not hypertension.
- Congenital anomalies are not a direct consequence of chronic hypertension.
Which of the following is a potential consequence of untreated maternal thyroid disorders during pregnancy?
Explanation
Maternal thyroid disorders in pregnancy alter metabolism, impair neurodevelopment, and disturb placental function. Thyroid hormones regulate fetal brain growth, myelination, and neuronal differentiation. Normal thyroid-stimulating hormone (TSH) in the 1st trimester is 0.1–2.5 mIU/L, free thyroxine (T4) is 0.8–1.7 ng/dL, and triiodothyronine (T3) is 80–200 ng/dL. Untreated hypothyroidism leads to impaired cognitive function, growth restriction, and miscarriage, while uncontrolled hyperthyroidism increases risk of preeclampsia, preterm birth, and fetal thyrotoxicosis.
Rationale for correct answer
3. Neurodevelopmental issues occur when maternal hypothyroidism causes inadequate transfer of thyroid hormones essential for fetal brain development. Deficiency during early gestation leads to intellectual impairment, reduced IQ, and motor delays. The lack of maternal thyroid hormone supply directly explains the fetal complications.
Rationale for incorrect answers
1. Placental abruption is mainly associated with chronic hypertension, preeclampsia, trauma, smoking, and cocaine use. Thyroid disorders do not directly damage placental attachment or cause premature separation.
2. Fetal alcohol spectrum disorder results from maternal alcohol consumption during pregnancy. It is characterized by growth restriction, facial anomalies, and neurobehavioral impairment, unrelated to thyroid hormone imbalance.
4. Gestational hypertension is defined as blood pressure ≥140/90 mmHg after 20 weeks without proteinuria. Thyroid dysfunction is not a primary cause. Hypertension is more linked to vascular or placental pathology, not thyroid hormone imbalance.
Take home points
- Maternal thyroid hormones are critical for fetal brain development, especially in early gestation.
- Untreated hypothyroidism increases risk of neurodevelopmental delay and reduced IQ in offspring.
- Hyperthyroidism in pregnancy causes maternal and fetal complications but not direct alcohol-like syndromes.
- Placental abruption and gestational hypertension are vascular disorders, not endocrine-driven thyroid effects.
Which of the following are potential fetal complications of poorly controlled gestational diabetes mellitus? Select all that apply.
Explanation
Gestational diabetes mellitus results from impaired glucose tolerance, maternal hyperglycemia, and excess fetal insulin production. Normal fasting plasma glucose is <100 mg/dL, 1-hour postprandial glucose should be <140 mg/dL, and 2-hour postprandial glucose should be <120 mg/dL in pregnancy. Poor control leads to fetal hyperinsulinemia, which acts as a growth-promoting hormone. This causes macrosomia, neonatal hypoglycemia, and obstetric complications such as shoulder dystocia. Intrauterine growth restriction is more associated with placental insufficiency from maternal hypertension. Congenital rubella syndrome results from viral infection, not hyperglycemia.
Rationale for correct answer
1. Macrosomia develops because maternal hyperglycemia increases glucose transfer to the fetus. Fetal pancreatic hyperinsulinemia stimulates fat deposition, leading to birth weight >4,000 g.
2. Neonatal hypoglycemia occurs after birth because the infant’s pancreas continues producing high insulin levels while maternal glucose supply is cut off. Normal neonatal glucose should be >45 mg/dL; values below this indicate hypoglycemia.
4. Shoulder dystocia is a direct consequence of macrosomia, where the fetal shoulder becomes impacted behind the maternal pubic symphysis during delivery. It is a recognized obstetric emergency in diabetic pregnancies.
Rationale for incorrect answers
3. Intrauterine growth restriction is not typical of gestational diabetes. It is more commonly linked to maternal hypertension, preeclampsia, or uteroplacental insufficiency, where fetal nutrient supply is limited.
5. Congenital rubella syndrome results from maternal rubella infection in the first trimester, leading to cataracts, deafness, and cardiac defects. It is unrelated to glucose metabolism or gestational diabetes.
Take home points
- Poorly controlled gestational diabetes causes macrosomia and related birth trauma.
- Neonatal hypoglycemia results from persistent fetal hyperinsulinemia after birth.
- Intrauterine growth restriction is linked to hypertension, not diabetes.
- Congenital rubella syndrome is viral, not metabolic in origin.
Which of the following are key nursing interventions for a patient with preeclampsia? Select all that apply.
Explanation
Preeclampsia is a hypertensive disorder of pregnancy characterized by elevated blood pressure, proteinuria, and end-organ dysfunction after 20 weeks gestation. Normal blood pressure is <120/80 mmHg, while preeclampsia is diagnosed at ≥140/90 mmHg with proteinuria ≥300 mg/24 h or protein/creatinine ratio ≥0.3. Severe cases may show thrombocytopenia (<100,000/µL), elevated liver enzymes (AST/ALT >40 U/L), or serum creatinine >1.1 mg/dL. Nursing care focuses on monitoring, seizure prevention, and maternal-fetal stabilization.
Rationale for correct answers
1. Monitoring blood pressure is essential because preeclampsia is defined by hypertension and progression to severe ranges (≥160/110 mmHg) increases maternal and fetal risk.
2. Administering magnesium sulfate is indicated to prevent seizures (eclampsia). Normal serum magnesium is 1.7–2.4 mg/dL, and therapeutic levels for seizure prophylaxis are 4–7 mEq/L.
4. Assessing for proteinuria is critical because preeclampsia involves abnormal renal glomerular permeability. Dipstick testing or 24-hour urine collection confirms diagnosis and severity.
5. Providing dietary counseling is important to maintain adequate protein and balanced nutrition. Sodium restriction is not generally recommended, but patients should avoid excessive processed foods and maintain hydration.
Rationale for incorrect answers
3. Encouraging ambulation is unsafe in preeclampsia. Patients are at risk of seizures, hypertension crises, and placental abruption. Bed rest or activity restriction is often recommended in severe cases to reduce maternal-fetal stress.
Take home points
- Preeclampsia is diagnosed after 20 weeks with hypertension and proteinuria or end-organ signs.
- Magnesium sulfate is the drug of choice for seizure prophylaxis.
- Proteinuria assessment is central to diagnosis and monitoring.
- Ambulation may increase risks; close monitoring and rest are safer strategies.
Practice Exercise 3
Which infection, if contracted during the first trimester, can lead to congenital rubella syndrome?
Explanation
Rubella infection in pregnancy is a viral illness that can severely impair fetal development, cause teratogenic effects, and damage multiple organ systems. The rubella virus crosses the placenta, especially in the first trimester when fetal organs are forming. Normal maternal rubella IgG titer >10 IU/mL indicates immunity, while non-immune mothers are at high risk. Congenital rubella syndrome leads to sensorineural deafness, cataracts, congenital heart disease, microcephaly, and growth restriction.
Rationale for correct answer
3. Rubella infection in the first trimester directly causes congenital rubella syndrome. Viral replication in fetal tissues disrupts organogenesis, leading to the classic triad of deafness, cataracts, and cardiac anomalies. The risk of fetal malformation is highest when infection occurs before 12 weeks.
Rationale for incorrect answers
1. Cytomegalovirus is the most common congenital viral infection and may cause microcephaly, periventricular calcifications, and sensorineural hearing loss, but it does not cause congenital rubella syndrome.
2. Toxoplasmosis, caused by Toxoplasma gondii, can result in chorioretinitis, hydrocephalus, and intracranial calcifications. It is transmitted through undercooked meat or cat feces exposure, not associated with congenital rubella syndrome.
4. Group B Streptococcus is a bacterial infection causing neonatal sepsis, pneumonia, and meningitis if transmitted during delivery. It does not produce teratogenic effects in utero and is unrelated to rubella.
Take home points
- Rubella infection in the first trimester causes congenital rubella syndrome.
- Classic triad: sensorineural deafness, cataracts, and congenital heart disease.
- Cytomegalovirus and toxoplasmosis cause congenital infections but not rubella syndrome.
- Group B Streptococcus leads to neonatal sepsis, not congenital malformations.
Which of the following sexually transmitted infections (STIs) is part of the TORCH infections that can lead to significant congenital anomalies?
Explanation
Syphilis is a sexually transmitted infection caused by Treponema pallidum that can cross the placenta and cause severe congenital anomalies, fetal death, and systemic disease. Normal maternal screening involves nontreponemal tests (VDRL, RPR) and treponemal-specific tests (FTA-ABS, TP-PA). Congenital syphilis may present with hepatosplenomegaly, anemia (hemoglobin <13 g/dL in neonates), rash, Hutchinson teeth, saddle nose, and sensorineural deafness. It is included in the TORCH infections (Toxoplasmosis, Other [syphilis], Rubella, Cytomegalovirus, Herpes simplex virus).
Rationale for correct answer
3. Syphilis is part of the “O” in TORCH and can cross the placenta at any stage of pregnancy. It causes congenital syphilis with manifestations such as hydrops fetalis, hepatomegaly, skin lesions, and later findings including Hutchinson teeth and saddle nose deformity.
Rationale for incorrect answers
1. Chlamydia is associated with neonatal conjunctivitis and pneumonia but is not classified under TORCH infections. It does not typically cause congenital structural anomalies.
2. Gonorrhea can cause ophthalmia neonatorum and neonatal sepsis but is not part of TORCH and does not produce congenital anomalies in utero.
4. Trichomoniasis causes preterm rupture of membranes, preterm birth, and low birth weight but does not cause congenital anomalies and is not part of TORCH.
Take home points
- TORCH infections include toxoplasmosis, syphilis, rubella, cytomegalovirus, and herpes simplex.
- Syphilis is the “O” in TORCH and causes congenital anomalies and stillbirth.
- Chlamydia, gonorrhea, and trichomoniasis cause neonatal infections but not TORCH anomalies.
- Screening and treatment of syphilis in pregnancy prevent congenital transmission.
What is the primary reason for administering prophylactic antibiotics during labor to a pregnant woman who tested positive for Group B Streptococcus (GBS)?
Explanation
Group B Streptococcus (GBS) is a gram-positive bacterium that colonizes the genitourinary tract and can be transmitted during vaginal delivery. Neonates exposed to GBS are at high risk of early-onset sepsis, pneumonia, and meningitis. Normal maternal screening is performed at 35–37 weeks of gestation using vaginal and rectal swabs. Prophylactic intravenous penicillin G or ampicillin during labor reduces neonatal infection risk without affecting uterine contractions, hemorrhage, or maternal long-term colonization.
Rationale for correct answer
2. The primary reason for giving intrapartum antibiotics in GBS-positive mothers is to prevent neonatal GBS sepsis, which can occur within the first 7 days of life and has a high mortality rate.
Rationale for incorrect answers
1. Prophylactic antibiotics are not given to prevent maternal postpartum infection. GBS rarely causes maternal endometritis or wound infection; the intervention specifically targets neonatal transmission.
3. Antibiotics do not influence coagulation pathways and therefore have no effect on maternal hemorrhage risk. Hemorrhage prevention relies on uterotonics and active management of the third stage of labor.
4. Antibiotics do not stimulate uterine contractions. Uterine contractility is managed with oxytocin, prostaglandins, or other uterotonics, not antimicrobial therapy.
Take home points
- GBS is screened for at 35–37 weeks of gestation.
- Main neonatal risks: sepsis, pneumonia, and meningitis.
- Intrapartum IV penicillin or ampicillin prevents early-onset neonatal disease.
- Maternal infection prevention is not the primary goal of prophylaxis.
Which of the following are potential neonatal complications of TORCH infections? Select all that apply.
Explanation
TORCH infections are a group of congenital infections including toxoplasmosis, other (syphilis, varicella-zoster, parvovirus B19), rubella, cytomegalovirus, and herpes simplex virus. They cross the placenta and cause teratogenic effects. Manifestations include microcephaly, hepatosplenomegaly, growth restriction, rash, and ocular abnormalities. Normal newborn head circumference is 32–36 cm. Normal glucose levels in neonates are 40–60 mg/dL within the first 24 hours.
Rationale for correct answer
1. Microcephaly occurs due to impaired brain development from intrauterine infection. Viral invasion such as cytomegalovirus and rubella directly damages neural progenitor cells, resulting in reduced cranial growth.
3. Sensorineural hearing loss is linked to cytomegalovirus and rubella infections. Viral damage to the inner ear structures and auditory nerve occurs in utero, leading to irreversible deficits after birth.
4. Congenital cataracts develop primarily from rubella infection. Viral replication in the developing lens leads to opacity formation and impaired visual development that can cause lifelong blindness if untreated.
Rationale for incorrect answers
2. Neonatal hypoglycemia is not a direct complication of TORCH infections. It is typically associated with maternal diabetes, intrauterine growth restriction, or perinatal stress. TORCH pathogens primarily cause structural anomalies and neurologic deficits rather than acute metabolic derangements.
5. Shoulder dystocia is a mechanical complication of labor, not an outcome of TORCH infections. It is caused by fetal macrosomia, maternal diabetes, or abnormal pelvic anatomy, not intrauterine infection. TORCH-related complications are congenital abnormalities, not intrapartum mechanical problems.
Take home points
- TORCH infections cross the placenta and damage fetal organs.
- Microcephaly, hearing loss, and cataracts are common complications.
- Hypoglycemia is metabolic, not infectious in origin.
- Shoulder dystocia is mechanical, not congenital.
Which of the following are key nursing interventions for preventing vertical transmission of infections during pregnancy? Select all that apply.
Explanation
Vertical transmission refers to the passage of infection from mother to fetus during pregnancy, labor, or delivery. Major pathogens include HIV, GBS, HSV, and TORCH organisms. Prevention strategies involve screening, prophylactic treatment, and safe delivery methods. Normal maternal blood glucose is 70–99 mg/dL fasting and 70–140 mg/dL postprandial, but glucose control does not directly prevent infection spread.
Rationale for correct answers
1. Administering penicillin for GBS-positive mothers during labor prevents neonatal sepsis, pneumonia, and meningitis by reducing bacterial colonization in the birth canal.
2. Educating patients on avoiding undercooked meat reduces toxoplasmosis risk, since Toxoplasma gondii cysts survive in raw or poorly cooked animal tissue and can cross the placenta.
3. Screening for HIV at the initial prenatal visit ensures early detection and initiation of antiretroviral therapy, which reduces mother-to-child transmission rates from about 25% to less than 2%.
5. Recommending cesarean delivery for active HSV lesions prevents neonatal exposure to herpes simplex virus during passage through the birth canal, thereby reducing risk of neonatal herpes infection.
Rationale for incorrect answers
4. Monitoring blood glucose levels is important for detecting gestational diabetes, but it does not prevent vertical transmission of infections. Hyperglycemia increases risk of macrosomia and neonatal hypoglycemia, but it is unrelated to infectious disease transmission from mother to fetus.
Take home points
- Vertical transmission occurs during pregnancy, labor, or delivery.
- Key prevention: maternal screening, prophylaxis, safe food handling, and delivery planning.
- HIV, HSV, GBS, and toxoplasmosis are major targets for prevention.
- Blood glucose monitoring prevents metabolic complications, not infection spread.
Practice Exercise 4
What is a potential consequence of maternal opioid use during pregnancy?
Explanation
Maternal opioid use causes neonatal abstinence syndrome due to chronic in-utero exposure to opioids such as heroin, methadone, or oxycodone. These drugs cross the placenta and stimulate fetal opioid receptors. After birth, the abrupt drug withdrawal leads to neurologic and autonomic symptoms including irritability, tremors, diarrhea, and seizures. Normal neonatal respiratory rate is 30–60 breaths/min, heart rate 120–160 beats/min, and glucose 40–60 mg/dL in the first 24 hours.
Rationale for correct answer
2. Neonatal abstinence syndrome develops because opioids readily cross the placenta and accumulate in the fetal system. Following delivery, drug supply is interrupted, causing withdrawal marked by hypertonia, high-pitched cry, and poor feeding. These symptoms are well recognized consequences of chronic maternal opioid use.
Rationale for incorrect answers
1. Fetal alcohol spectrum disorder results from maternal alcohol consumption, not opioid use. Alcohol causes microcephaly, smooth philtrum, thin upper lip, and growth retardation, whereas opioids cause withdrawal, not teratogenic facial abnormalities.
3. Congenital heart defects are most often associated with chromosomal abnormalities (e.g., trisomy 21) or maternal rubella infection. Opioid exposure does not directly interfere with cardiac septation or outflow tract formation, making this an incorrect consequence.
4. Placenta previa is linked to uterine scarring, multiparity, and advanced maternal age. It occurs when the placenta implants over the cervical os, unrelated to opioid exposure. Opioids act pharmacologically, not structurally, and do not determine placental implantation sites.
Take home points
- Maternal opioid use leads to neonatal abstinence syndrome after delivery.
- Symptoms include tremors, irritability, diarrhea, seizures, and feeding difficulty.
- Alcohol, not opioids, causes fetal alcohol spectrum disorder.
- Placenta previa and congenital heart defects are unrelated to opioid use.
Which environmental exposure is most likely to increase the risk of miscarriage and developmental delays in the fetus?
Explanation
Lead exposure in pregnancy causes miscarriage and neurodevelopmental delays due to its ability to cross the placenta and accumulate in fetal tissues. Lead interferes with neurologic development by disrupting synapse formation and neurotransmitter release. Blood lead levels should be <5 µg/dL in children and <10 µg/dL in adults to be considered safe. Elevated levels increase risks of low birth weight, preterm delivery, and impaired cognition.
Rationale for correct answer
2. Lead exposure is strongly linked to miscarriage and developmental delays. Lead crosses the placenta and accumulates in fetal bone and brain tissue. This results in impaired synaptogenesis, neuronal death, and long-term cognitive deficits. Miscarriage is associated with maternal blood lead levels >10 µg/dL.
Rationale for incorrect answers
1. Mercury exposure, particularly methylmercury from contaminated fish, primarily causes neurotoxicity manifesting as impaired motor skills, cerebral palsy-like features, and sensory deficits. While harmful, it is less directly associated with miscarriage compared to lead.
3. Air pollution increases risks of low birth weight, intrauterine growth restriction, and preterm birth, but evidence for miscarriage and direct neurodevelopmental delay is weaker compared to lead exposure. The impact is more related to hypoxia and placental inflammation.
4. Radiation, especially doses >100 mGy, is associated with growth restriction, microcephaly, and increased childhood cancer risk. However, miscarriage and developmental delay are more consistently documented with lead exposure at much lower levels, making radiation less likely in this context.
Take home points
- Lead exposure in pregnancy is a major cause of miscarriage and developmental delay.
- Mercury mainly causes neurotoxicity and motor deficits, not miscarriage.
- Air pollution contributes to low birth weight and preterm birth.
- Radiation at high doses causes growth restriction and microcephaly, but not primarily miscarriage.
Which maternal behavior is most associated with sudden infant death syndrome (SIDS)?
Explanation
Sudden infant death syndrome (SIDS) is the unexplained death of an infant under 1 year, usually during sleep. Maternal smoking is the strongest modifiable risk factor, as nicotine causes impaired autonomic regulation, reduced arousal, and hypoxemia in the fetus. Normal infant respiratory rate is 30–60 breaths/min and heart rate is 120–160 beats/min; disruption of autonomic control increases risk of apnea and death during sleep.
Rationale for correct answer
2. Smoking during pregnancy and after delivery exposes the fetus and infant to nicotine and carbon monoxide. This reduces oxygen delivery, impairs brainstem respiratory control, and increases SIDS risk by 2–4 times compared to nonsmoking mothers.
Rationale for incorrect answers
1. Caffeine consumption during pregnancy is linked to low birth weight and miscarriage at high intake (>200–300 mg/day), but it is not a major contributor to SIDS. It does not significantly impair neonatal respiratory regulation.
3. High sugar intake predisposes to gestational diabetes and macrosomia but has no established link to sudden infant death. It affects metabolic health, not respiratory arousal mechanisms.
4. Sedentary lifestyle increases maternal obesity risk and complications such as preeclampsia and gestational diabetes, but it does not directly cause SIDS. Infant mortality in this case is more related to perinatal complications than unexplained sudden death.
Take home points
- SIDS is unexplained infant death under 1 year, often during sleep.
- Maternal smoking is the strongest modifiable risk factor.
- Caffeine and high sugar intake affect pregnancy outcomes but not SIDS.
- Sedentary lifestyle increases maternal risk, not infant sudden death.
Which of the following are potential fetal complications of maternal alcohol consumption during pregnancy? Select all that apply.
Explanation
Fetal alcohol spectrum disorder (FASD) results from maternal alcohol consumption during pregnancy. Ethanol freely crosses the placenta and interferes with neurodevelopment, cellular differentiation, and organogenesis. Manifestations include facial dysmorphia, intrauterine growth restriction, and lifelong cognitive impairment. Normal birth weight is 2.5–4.0 kg, and growth below the 10th percentile indicates restriction. There is no safe threshold of alcohol during pregnancy.
Rationale for correct answer
1. Facial dysmorphia occurs due to alcohol-induced disruption of neural crest cell migration. Classic features include smooth philtrum, thin upper lip, and short palpebral fissures.
3. Growth restriction results from impaired placental blood flow and direct fetal toxicity of ethanol, leading to birth weights <2.5 kg or below the 10th percentile.
4. Neurodevelopmental deficits are long-term consequences of prenatal alcohol exposure, presenting as poor memory, attention deficits, and reduced IQ, caused by neuronal apoptosis and abnormal brain development.
Rationale for incorrect answers
2. Neonatal abstinence syndrome is due to maternal opioid use, not alcohol exposure. It results from withdrawal after delivery and manifests with tremors, irritability, and seizures, unlike structural teratogenic effects of alcohol.
5. Congenital rubella syndrome is caused by maternal rubella infection, not alcohol. It is characterized by cataracts, sensorineural hearing loss, and congenital heart defects, which are unrelated to ethanol teratogenicity.
Take home points
- Alcohol in pregnancy causes fetal alcohol spectrum disorder.
- Classic features: facial dysmorphia, growth restriction, and lifelong neurodevelopmental impairment.
- Opioids, not alcohol, cause neonatal abstinence syndrome.
- Rubella infection, not alcohol, causes congenital rubella syndrome.
Which of the following are key nursing interventions for managing environmental toxin exposure during pregnancy? Select all that apply.
Explanation
Environmental toxin exposure in pregnancy poses risks of miscarriage, growth restriction, and neurodevelopmental deficits. Substances such as mercury, lead, air pollutants, and radiation cross the placenta and disrupt fetal organogenesis. Safe maternal blood lead level is <10 µg/dL, while methylmercury intake should be <0.1 µg/kg/day. Radiation exposure >100 mGy increases fetal malformation risk.
Rationale for correct answer
1. Educating patients to avoid high-mercury fish such as shark, swordfish, and king mackerel prevents methylmercury accumulation, which causes fetal neurotoxicity and cerebral palsy-like features.
3. Screening for lead exposure is critical in high-risk groups (old housing, contaminated water, occupational exposure). Lead crosses the placenta, and maternal levels >10 µg/dL are associated with miscarriage and cognitive impairment.
5. Advising limited radiographic imaging minimizes unnecessary fetal radiation exposure. Doses above 100 mGy increase risk of microcephaly, growth restriction, and childhood cancer, making judicious imaging essential.
Rationale for incorrect answers
2. Encouraging outdoor activities in high-pollution areas increases maternal exposure to particulate matter and carbon monoxide. These impair placental oxygen delivery and raise risks of preterm birth and low birth weight, making this harmful rather than protective.
4. Administering folic acid prevents neural tube defects but does not counteract mercury, lead, or radiation toxicity. Its mechanism is through DNA synthesis and repair, not detoxification, so it is not an intervention against environmental toxins.
Take home points
- Mercury, lead, and radiation are major teratogens during pregnancy.
- Avoiding high-mercury fish prevents fetal neurotoxicity.
- Screening for lead exposure reduces miscarriage and neurodevelopmental risk.
- Limiting radiation exposure is essential to prevent fetal malformations.
Practice Exercise 5
A nurse is conducting a psychosocial assessment. Which condition is a significant psychosocial risk factor for poor pregnancy outcomes?
Explanation
Intimate partner violence is a major psychosocial risk factor during pregnancy. It is linked to increased maternal morbidity, poor fetal outcomes, and heightened risk of depression. Pregnant women experiencing violence often present with low birth weight infants (<2500 g), preterm birth (<37 weeks), and maternal complications such as anemia (Hb <12 g/dL) and hypertension (normal <120/80 mmHg). Chronic stress from abuse increases cortisol, leading to placental dysfunction and impaired fetal growth.
Rationale for correct answer
2. Intimate partner violence is a psychosocial condition that significantly increases the risk of poor pregnancy outcomes. It causes maternal stress, depression, inadequate prenatal care, and substance use, all of which increase fetal morbidity and mortality. The question specifically asks for a psychosocial factor, making this the correct choice.
Rationale for incorrect answers
1. Gestational diabetes is a medical, not psychosocial, condition. It is associated with fetal macrosomia (>4000 g), neonatal hypoglycemia (normal 40–60 mg/dL), and polyhydramnios. However, it does not represent a psychosocial factor, and thus it is not the correct answer to this question.
3. Placental abruption is a direct obstetric complication caused by premature separation of the placenta, leading to hemorrhage and fetal distress. It presents with abdominal pain, vaginal bleeding, and non-reassuring fetal heart tones (normal baseline 110–160 bpm). It is a physical complication, not a psychosocial risk factor.
4. Multiple gestation is a biological condition that increases obstetric risk such as preterm birth, preeclampsia, and intrauterine growth restriction. It does not fall under psychosocial factors, which relate to social, emotional, or behavioral conditions affecting pregnancy outcomes.
Take home points
- Intimate partner violence is a psychosocial risk factor that worsens pregnancy outcomes.
- Gestational diabetes and multiple gestation are biological risk factors, not psychosocial.
- Placental abruption is an obstetric complication, not psychosocial in nature.
- Psychosocial risk factors influence maternal mental health and indirectly impact fetal well-being.
What is a key nursing intervention for a patient with suspected intimate partner violence during pregnancy?
Explanation
Intimate partner violence during pregnancy requires screening, privacy, and appropriate referral for safety. Violence leads to maternal stress, trauma, poor prenatal care, and adverse fetal outcomes like preterm birth (<37 weeks) and low birth weight (<2500 g). Nurses must conduct private, sensitive questioning because disclosure is unlikely in the presence of the abuser. Universal screening is recommended during prenatal visits to ensure early detection and intervention.
Rationale for correct answer
2. Conducting private, sensitive screening is the essential nursing intervention for suspected intimate partner violence. Asking nonjudgmental questions in a safe, confidential environment allows accurate identification of abuse and timely referral to resources, thereby reducing maternal and fetal morbidity.
Rationale for incorrect answers
1. Administering magnesium sulfate is indicated for preeclampsia or eclampsia to prevent seizures. It has no role in identifying or addressing intimate partner violence, which requires psychosocial screening and intervention.
3. Ordering a non-stress test evaluates fetal well-being by monitoring fetal heart rate (normal baseline 110–160 bpm). While useful in obstetric complications, it does not address the underlying psychosocial risk factor of intimate partner violence.
4. Monitoring blood glucose levels is appropriate for gestational diabetes management. Normal fasting glucose in pregnancy is <95 mg/dL, but this is unrelated to psychosocial risk factors such as intimate partner violence.
Take home points
- Intimate partner violence requires private, sensitive screening during prenatal visits.
- Magnesium sulfate is used for eclampsia, not psychosocial risks.
- Non-stress tests assess fetal well-being but do not screen for abuse.
- Glucose monitoring is specific to gestational diabetes, not intimate partner violence.
A nurse is monitoring a patient with multiple gestation. What is a common complication associated with twin pregnancies?
Explanation
Multiple gestation increases uterine distension, raising the risk of preterm birth, preeclampsia, and intrauterine growth restriction. In twin pregnancies, preterm birth is the most frequent complication, with over 50% delivering before 37 weeks (normal term = 37–42 weeks). Neonates born preterm face risks including respiratory distress syndrome due to surfactant deficiency (normal surfactant adequate after 34–36 weeks) and feeding difficulties. Increased placental demands also predispose to anemia (Hb <12 g/dL) and gestational hypertension (normal BP <120/80 mmHg).
Rationale for correct answer
2. Preterm birth is the most common complication of multiple gestation. Uterine overdistension, hormonal changes, and increased uteroplacental insufficiency lead to early labor, often before 37 weeks, making this the correct answer.
Rationale for incorrect answers
1. Postpartum depression is a psychosocial condition associated with hormonal shifts, lack of support, and stress. While it may occur after any pregnancy, it is not a direct obstetric complication unique to multiple gestation.
3. Neural tube defects such as spina bifida occur due to folate deficiency and genetic predisposition. They are not more common specifically because of multiple gestation, though nutritional demands are higher in twin pregnancies.
4. Congenital rubella syndrome results from maternal rubella infection during pregnancy. It is unrelated to multiple gestation and presents with deafness, cataracts, and congenital heart disease, not a complication caused by twin pregnancy.
Take home points
- Multiple gestation most commonly leads to preterm birth due to uterine overdistension.
- Neural tube defects are linked to folate deficiency, not multiple gestation itself.
- Congenital rubella syndrome results from maternal infection, not twin pregnancy.
- Postpartum depression is psychosocial, not a direct complication of multiple gestation.
Which of the following are potential consequences of intimate partner violence during pregnancy? Select all that apply.
Explanation
Intimate partner violence during pregnancy causes physical trauma, chronic stress, and inadequate prenatal care, leading to serious maternal and fetal complications. Trauma increases catecholamines and cortisol, impairing uteroplacental perfusion and raising the risk of miscarriage (loss before 20 weeks), preterm labor (<37 weeks), and placental abruption (premature placental separation). These complications stem from both direct physical injury and indirect physiologic stress. Normal pregnancy carries miscarriage risk of 10–15%, but this risk rises with violence. Placental abruption can cause hypovolemia and fetal distress (normal FHR baseline 110–160 bpm).
Rationale for correct answer
1. Miscarriage can occur from direct abdominal trauma or severe maternal stress. Disruption of placental attachment and uterine perfusion contributes to early pregnancy loss.
2. Preterm labor is strongly associated with intimate partner violence. Stress hormones and trauma can trigger uterine contractions, leading to delivery before 37 weeks gestation.
3. Placental abruption results from trauma to the abdomen or chronic vascular stress, leading to premature placental separation. This causes vaginal bleeding, abdominal pain, and compromised fetal oxygenation.
Rationale for incorrect answers
4. Fetal macrosomia (>4000 g) is usually associated with gestational diabetes due to maternal hyperglycemia and increased fetal insulin production. It is not a complication of intimate partner violence.
5. Neonatal hypoglycemia (plasma glucose <40 mg/dL in the first 24 hours) is also linked to infants of diabetic mothers due to excess insulin production in utero. It does not result from intimate partner violence.
Take home points
- Intimate partner violence can cause miscarriage, preterm labor, and placental abruption.
- Fetal macrosomia is associated with gestational diabetes, not abuse.
- Neonatal hypoglycemia is linked to maternal diabetes, not trauma.
- Trauma and stress responses are key mechanisms of violence-related pregnancy complications.
Which of the following are obstetric complications associated with placental abnormalities? Select all that apply.
Explanation
Placental abnormalities such as placenta previa and placental abruption compromise maternal perfusion, cause hemorrhage, and impair oxygen and nutrient transfer to the fetus. These conditions lead to maternal hemorrhage (normal pregnancy blood loss at delivery <500 mL vaginal, <1000 mL cesarean), fetal hypoxia due to disrupted placental oxygen exchange (normal umbilical venous PO₂ ≈ 30 mmHg), and preterm birth (<37 weeks) due to uteroplacental instability. Placental pathology does not directly cause metabolic conditions like neonatal hypoglycemia or congenital structural anomalies like heart defects.
Rationale for correct answers
1. Maternal hemorrhage occurs with placental abruption and placenta previa, where separation or abnormal implantation of the placenta leads to severe bleeding.
2. Fetal hypoxia results from impaired maternal-fetal oxygen transfer when the placenta detaches or is compromised, leading to abnormal fetal heart rate patterns.
4. Preterm birth is common with placental abnormalities, often due to bleeding, uteroplacental insufficiency, or iatrogenic delivery to save maternal or fetal life.
Rationale for incorrect answers
3. Neonatal hypoglycemia (plasma glucose <40 mg/dL in first 24 hours) is most commonly seen in infants of diabetic mothers or those with intrauterine growth restriction. It is not directly caused by placental abnormalities.
5. Congenital heart defects are structural anomalies from abnormal embryonic development, often linked to genetic syndromes or teratogens like rubella. They are not a complication of placental pathology.
Take home points
- Placental abnormalities cause maternal hemorrhage, fetal hypoxia, and preterm birth.
- Neonatal hypoglycemia is metabolic, often due to maternal diabetes.
- Congenital heart defects result from embryonic malformations, not placental disorders.
- Placenta previa and abruption threaten both maternal and fetal survival.
Practice Exercise 6
A nurse is educating a pregnant patient about nutritional deficiencies. Which nutrient deficiency is most associated with neural tube defects?
Explanation
Folic acid deficiency is the primary cause of neural tube defects such as spina bifida and anencephaly. Neural tube closure normally occurs by day 28 of embryonic development, often before pregnancy is recognized. Adequate folate levels (normal serum folate >4 ng/mL) are essential for DNA synthesis, cell division, and neural tissue development. Women of childbearing age are advised to take 400–800 µg daily to prevent defects, with higher doses (up to 4 mg/day) recommended for those with prior affected pregnancies.
Rationale for correct answer
3. Folic acid deficiency impairs neural tube closure due to defective DNA synthesis and cell division. This leads to structural anomalies like spina bifida, making it the most associated nutrient deficiency.
Rationale for incorrect answers
1. Iron deficiency causes maternal anemia (Hb <12 g/dL in pregnancy) leading to fatigue, pallor, and low birth weight infants, but it is not linked to neural tube defects.
2. Calcium deficiency increases maternal risk of osteopenia and may contribute to hypertensive disorders of pregnancy. It does not affect neural tube development.
4. Vitamin D deficiency leads to maternal bone demineralization and neonatal rickets. It is not associated with neural tube closure failure.
Take home points
- Folic acid deficiency is the key cause of neural tube defects.
- Iron deficiency causes maternal anemia but not neural tube defects.
- Calcium deficiency contributes to bone and blood pressure complications, not neural tube defects.
- Vitamin D deficiency affects bone health but not neural tube closure.
What is a common consequence of food insecurity during pregnancy?
Explanation
Food insecurity during pregnancy leads to poor fetal growth due to inadequate caloric intake, micronutrient deficiencies, and chronic maternal stress. Restricted maternal nutrition reduces uteroplacental blood flow and nutrient transfer, resulting in intrauterine growth restriction (IUGR). IUGR is defined as estimated fetal weight below the 10th percentile for gestational age, with normal birth weight expected between 2500–4000 g at term. Infants affected are at risk of hypothermia, hypoglycemia (<40 mg/dL in first 24 hours), and long-term developmental delays.
Rationale for correct answer
2. Poor fetal growth occurs when maternal malnutrition and food insecurity limit nutrient supply. This results in IUGR and low birth weight, which are common outcomes of inadequate prenatal nutrition.
Rationale for incorrect answers
1. Macrosomia (>4000 g) is typically linked to gestational diabetes mellitus, not food insecurity, as it results from maternal hyperglycemia and excess fetal insulin.
3. Placenta previa, characterized by implantation of the placenta over the cervical os, is an anatomical abnormality unrelated to maternal nutrition or food insecurity.
4. Congenital heart defects arise from abnormal embryonic development influenced by genetic or teratogenic factors. They are not directly caused by food insecurity.
Take home points
- Food insecurity in pregnancy most often leads to poor fetal growth and low birth weight.
- Macrosomia results from maternal diabetes, not malnutrition.
- Placenta previa is a placental implantation disorder, not nutrition-related.
- Congenital heart defects are embryonic malformations, not outcomes of food insecurity.
Which of the following are potential complications associated with maternal obesity during pregnancy? Select all that apply.
Explanation
Maternal obesity in pregnancy is linked to insulin resistance, altered placental function, and systemic inflammation, which increase risks of multiple complications. Obesity raises the likelihood of gestational diabetes mellitus, preeclampsia, cesarean birth, and macrosomia (>4000 g). Neonates face higher risks of shoulder dystocia during delivery, hypoglycemia (<40 mg/dL in first 24 hours), and later-life metabolic disease. Stillbirth risk is elevated in obese pregnancies due to impaired uteroplacental perfusion, with obesity classified as a significant modifiable maternal risk factor.
Rationale for correct answers
1. Increased risk of gestational diabetes mellitus occurs because maternal obesity causes insulin resistance and hyperglycemia, leading to abnormal glucose tolerance.
3. Higher incidence of shoulder dystocia occurs due to fetal macrosomia, common in pregnancies complicated by maternal obesity and diabetes.
5. Increased risk of preeclampsia is linked to obesity through vascular dysfunction, endothelial injury, and chronic inflammation, which raise maternal blood pressure above normal levels (<120/80 mmHg).
Rationale for incorrect answers
2. Decreased risk of macrosomia is incorrect because obesity increases, not decreases, the likelihood of fetal overgrowth due to excess maternal glucose and fat transfer.
4. Reduced likelihood of stillbirth is incorrect because obesity increases stillbirth risk. Mechanisms include impaired placental function, chronic hypoxia, and metabolic abnormalities.
Take home points
- Maternal obesity raises risk for gestational diabetes, preeclampsia, macrosomia, and shoulder dystocia.
- Obesity is linked to increased stillbirth risk, not reduced.
- Macrosomia (>4000 g) is a hallmark complication, not less common in obesity.
- Insulin resistance and inflammation are central mechanisms of obesity-related pregnancy risks.
Which of the following are key nursing interventions for managing nutritional deficiencies in pregnancy? Select all that apply.
Explanation
Nutritional deficiencies in pregnancy increase risks of anemia, neural tube defects, poor fetal growth, and maternal complications. Iron deficiency anemia is the most common, defined as hemoglobin <11 g/dL in the 1st and 3rd trimesters, <10.5 g/dL in the 2nd trimester. Folate deficiency raises neural tube defect risk, prevented by 400–800 µg daily folic acid supplementation. Adequate dietary counseling on iron-rich foods (meat, legumes, leafy greens) improves hemoglobin and hematocrit (normal Hct in pregnancy: 33–44%). Food insecurity is a major barrier to adequate nutrition, requiring referrals to programs like WIC.
Rationale for correct answer
1. Educating on daily folic acid supplementation prevents neural tube defects by supporting DNA synthesis and neural tissue development.
2. Monitoring hemoglobin levels detects iron deficiency anemia early, allowing timely intervention to prevent maternal fatigue and fetal growth restriction.
4. Providing dietary counseling for iron-rich foods ensures adequate iron intake, enhancing hemoglobin production and preventing anemia-related complications.
5. Referring to WIC supports food-insecure patients by providing access to nutrient-rich foods, reducing risks of growth restriction and preterm birth.
Rationale for incorrect answers
3. Encouraging high-sugar diets is inappropriate. Excess sugar intake increases risk of gestational diabetes, macrosomia (>4000 g), and neonatal hypoglycemia (<40 mg/dL). It does not correct nutritional deficiencies.
Take home points
- Folic acid supplementation prevents neural tube defects.
- Iron deficiency anemia is common; monitor hemoglobin and diet.
- Food insecurity requires nursing referrals to support programs like WIC.
- High-sugar diets increase risks of gestational diabetes, not correct deficiencies.
Comprehensive Questions
A pregnant woman at 28 weeks gestation is Rh-negative and has no antibodies. Which of the following interventions is most appropriate for the nurse to anticipate?
Explanation
Rh incompatibility occurs when an Rh-negative mother carries an Rh-positive fetus. Hemolysis results from maternal IgG antibodies crossing the placenta and destroying fetal red blood cells. Anemia and hydrops fetalis may occur in severe cases. Normal maternal hemoglobin is 12–16 g/dL and hematocrit is 36–46%. Antibody screening is performed at the first prenatal visit and repeated at 28 weeks if the mother is Rh-negative. Prophylaxis with anti-D immunoglobulin prevents maternal sensitization and protects future pregnancies.
Rationale for correct answer
2. Administration of RhoGAM at 28 weeks is standard to prevent maternal alloimmunization. The drug binds fetal Rh-positive cells that may enter maternal circulation before antibodies form. This prophylaxis decreases risk of hemolytic disease of the newborn in subsequent pregnancies.
Rationale for incorrect answers
1. Hepatitis B vaccine is not indicated in this situation unless the mother is unvaccinated or high risk. It does not prevent alloimmunization and is unrelated to Rh factor status. The timing of hepatitis B vaccination is independent of maternal Rh typing.
3. Screening for Group B Streptococcus is done between 35–37 weeks, not at 28 weeks. The rationale is that colonization status can change during pregnancy, so screening earlier may not provide accurate guidance for intrapartum prophylaxis.
4. Pap smears are not indicated routinely during pregnancy unless cervical screening is due. Performing a Pap smear at 28 weeks offers no benefit in preventing Rh incompatibility and is not part of standard prenatal care at this gestational age.
Take home points
- RhoGAM is given at 28 weeks and within 72 hours postpartum if the baby is Rh-positive.
- Hemolytic disease of the newborn results from maternal IgG antibodies destroying fetal red blood cells.
- GBS screening is done at 35–37 weeks, not 28 weeks.
- Hepatitis B vaccination is unrelated to Rh alloimmunization prevention.
What is the primary purpose of measuring fundal height after 20 weeks gestation?
Explanation
Fundal height measurement is a simple clinical tool used in pregnancy to monitor fetal growth and estimate gestational age after 20 weeks. From 20–36 weeks, the fundal height in centimeters usually corresponds to gestational age in weeks ±2 cm. Normal amniotic fluid index is 8–24 cm. Deviations may suggest intrauterine growth restriction (IUGR) or macrosomia. Measurement reliability decreases after 36 weeks due to fetal descent.
Rationale for correct answer
3. After 20 weeks, fundal height in centimeters approximates gestational age in weeks, allowing assessment of fetal growth and amniotic fluid volume. This provides a non-invasive method for monitoring intrauterine development.
Rationale for incorrect answers
1. Fundal height does not directly assess maternal weight gain. Maternal weight gain is measured using a scale, and normal recommended gain for a woman with normal BMI is 11.5–16 kg.
2. Determination of fetal lie and presentation is done using Leopold’s maneuvers and ultrasound, not fundal height measurement. Fundal height does not indicate fetal orientation.
4. Fetal heart rate variability is monitored using electronic fetal monitoring or Doppler auscultation, not fundal height. Variability is assessed in beats per minute and reflects fetal well-being.
Take home points
- Fundal height correlates with gestational age between 20–36 weeks within ±2 cm.
- Deviations may indicate IUGR, macrosomia, or abnormal amniotic fluid volume.
- Leopold’s maneuvers, not fundal height, assess fetal lie and presentation.
- Fetal heart rate variability requires electronic monitoring, not abdominal measurement.
A pregnant patient reports experiencing severe, persistent headaches and blurred vision at 34 weeks gestation. Which of the following conditions should the nurse immediately suspect?
Explanation
Preeclampsia is a hypertensive disorder of pregnancy characterized by hypertension, proteinuria, and potential end-organ dysfunction after 20 weeks gestation. Normal blood pressure in pregnancy is <140/90 mmHg. Proteinuria is significant if ≥300 mg/24 h. Severe features include systolic ≥160 mmHg or diastolic ≥110 mmHg, thrombocytopenia <100,000/µL, creatinine >1.1 mg/dL, and symptoms such as headache or visual disturbances. Complications include eclampsia, HELLP syndrome, and fetal growth restriction.
Rationale for correct answer
3. Severe, persistent headaches and blurred vision at 34 weeks are classic signs of preeclampsia with severe features. These result from cerebral vasospasm and edema due to endothelial dysfunction, requiring urgent evaluation and management to prevent seizures.
Rationale for incorrect answers
1. Gestational diabetes mellitus usually presents with polyuria, polydipsia, and polyphagia due to glucose intolerance. It is diagnosed with an oral glucose tolerance test. It does not cause neurologic symptoms such as headaches and blurred vision.
2. Iron deficiency anemia presents with fatigue, pallor, tachycardia, and low hemoglobin (<11 g/dL in pregnancy). It does not cause hypertension, headache, or visual disturbances.
4. Urinary tract infection in pregnancy typically causes dysuria, frequency, urgency, and suprapubic discomfort. Severe cases may cause flank pain or fever but not hypertension with neurologic symptoms.
Take home points
- Preeclampsia is defined as hypertension after 20 weeks with proteinuria or end-organ dysfunction.
- Severe features include headache, visual changes, thrombocytopenia, renal impairment, and liver dysfunction.
- Complications include eclampsia, HELLP syndrome, and intrauterine growth restriction.
- Differentiation from gestational diabetes, anemia, and UTI relies on symptom-specific clinical findings.
Which of the following is a key component of the First Trimester Screening for aneuploidies?
Explanation
First trimester screening is used to assess the risk of aneuploidy such as trisomy 21 and trisomy 18 between 11–14 weeks gestation. It combines nuchal translucency ultrasound measurement with maternal serum levels of free β-hCG and PAPP-A. Increased nuchal translucency (>3.0 mm) is associated with chromosomal abnormalities and congenital heart disease. Normal crown-rump length is used to standardize NT measurements. This screening provides early risk stratification before diagnostic testing.
Rationale for correct answer
3. Nuchal translucency ultrasound is a standard component of first trimester aneuploidy screening. Increased NT thickness indicates abnormal lymphatic fluid accumulation in the fetal neck, often associated with trisomy 21, trisomy 18, and congenital heart defects.
Rationale for incorrect answers
1. Maternal serum alpha-fetoprotein is part of the second trimester quadruple screen, not the first trimester screen. Elevated levels are linked to neural tube defects, while low levels may indicate trisomy 21.
2. The one-hour glucose tolerance test is performed between 24–28 weeks gestation to screen for gestational diabetes mellitus. It is not used for aneuploidy risk assessment.
4. Group B Streptococcus culture is performed at 35–37 weeks to identify carriers who require intrapartum antibiotic prophylaxis. It has no role in first trimester aneuploidy screening.
Take home points
- First trimester screening combines NT ultrasound, free β-hCG, and PAPP-A.
- Increased NT thickness (>3.0 mm) suggests aneuploidy and congenital heart disease.
- Second trimester screening uses MSAFP, hCG, estriol, and inhibin A.
- GTT and GBS culture are performed later in pregnancy for different purposes.
A nurse is counseling a pregnant patient on nutrition. Which of the following food items should the nurse advise the patient to avoid due to potential risks?
Explanation
Foodborne infections in pregnancy increase risk of maternal illness and fetal complications due to immunologic changes. Pregnant women are more susceptible to pathogens such as Listeria monocytogenes, Toxoplasma gondii, and Salmonella. Raw seafood may harbor parasites, viruses, and bacteria, posing risks of miscarriage, stillbirth, or neonatal sepsis. Normal maternal white blood cell count in pregnancy is 6,000–16,000/µL due to physiologic leukocytosis. Safe food handling, cooking meat to ≥74°C, and avoiding unpasteurized products are essential preventive measures.
Rationale for correct answer
3. Raw sushi should be avoided because it may contain Listeria and other pathogens. Infection during pregnancy can cross the placenta and cause miscarriage, stillbirth, or neonatal sepsis. Cooking eliminates these risks, making raw seafood unsafe.
Rationale for incorrect answers
1. Cooked chicken breast is safe in pregnancy if thoroughly cooked to an internal temperature of ≥74°C. Proper cooking destroys harmful pathogens such as Salmonella and Campylobacter.
2. Pasteurized milk is safe because pasteurization kills Listeria and other harmful bacteria. Unpasteurized milk, not pasteurized, poses infection risks in pregnancy.
4. Fully cooked vegetables are safe and provide essential vitamins, minerals, and fiber. Risk occurs only with raw or improperly washed vegetables that may harbor Toxoplasma gondii oocysts.
Take home points
- Raw sushi and undercooked seafood should be avoided in pregnancy due to infection risks.
- Pasteurized dairy products are safe; unpasteurized products should be avoided.
- Thorough cooking of meat to ≥74°C prevents bacterial foodborne illness.
- Raw unwashed vegetables may carry Toxoplasma; cooking ensures safety.
When discussing warning signs with a pregnant patient, which of the following symptoms should be emphasized as requiring immediate medical attention?
Explanation
Preterm premature rupture of membranes (PPROM) involves fluid leakage from the vagina due to rupture of the amniotic sac before labor. Amniotic fluid normally ranges from 500–1,000 mL at term, with an amniotic fluid index (AFI) of 8–24 cm. Leakage increases risk of infection, preterm birth, and umbilical cord prolapse. Differentiating true amniotic fluid leakage from urinary incontinence can be confirmed with tests such as nitrazine (alkaline pH) and ferning. Prompt medical evaluation is critical for maternal-fetal safety.
Rationale for correct answer
3. Fluid leakage from the vagina is a danger sign because it suggests rupture of membranes, which places the fetus at risk for infection and cord prolapse. Immediate medical evaluation is necessary to prevent complications.
Rationale for incorrect answers
1. Mild ankle swelling is common in late pregnancy due to increased venous pressure and fluid retention. It is generally benign unless associated with hypertension or proteinuria, which would suggest preeclampsia.
2. Occasional heartburn results from progesterone-induced relaxation of the lower esophageal sphincter and uterine pressure on the stomach. While uncomfortable, it is not a warning sign.
4. Fatigue is a common symptom throughout pregnancy due to hormonal changes, increased metabolic demands, and anemia risk. It does not require urgent evaluation unless severe or associated with other symptoms.
Take home points
- Vaginal fluid leakage is an urgent warning sign requiring immediate evaluation.
- Mild ankle swelling and heartburn are common discomforts, not emergencies.
- Fatigue is expected in pregnancy but should be monitored if severe or persistent.
- Ruptured membranes increase risk of infection, preterm labor, and cord prolapse.
Which of the following statements about alcohol consumption during pregnancy is most accurate?
Explanation
Alcohol consumption in pregnancy poses significant risks because ethanol freely crosses the placenta, and the fetal liver cannot metabolize it effectively. This leads to fetal alcohol spectrum disorders (FASD), characterized by growth restriction, neurodevelopmental delay, and craniofacial abnormalities. There is no safe threshold; even small amounts can disrupt organogenesis. Normal newborn head circumference is 32–36 cm, but alcohol exposure may cause microcephaly. Abstinence from alcohol is the only proven preventive measure.
Rationale for correct answer
3. There is no known safe amount of alcohol consumption in pregnancy. Any exposure increases risk of teratogenic effects, especially during the first trimester when organogenesis occurs, but risks persist throughout gestation.
Rationale for incorrect answers
1. Moderate alcohol consumption after the first trimester is not safe. The fetal brain continues developing throughout pregnancy, and alcohol can still cause growth and neurodevelopmental impairment.
2. It is not only heavy drinking that causes harm; even light or occasional drinking has been linked to subtle cognitive and behavioral issues. No level is safe.
4. Red wine is not permissible in pregnancy, regardless of its antioxidant content. The ethanol in wine carries the same teratogenic risks as other alcoholic beverages.
Take home points
- No amount of alcohol is safe at any point in pregnancy.
- Alcohol causes fetal alcohol spectrum disorders with lifelong effects.
- Both heavy and light alcohol use can impair fetal growth and brain development.
- Prevention requires total abstinence during pregnancy.
A pregnant patient with pre-gestational diabetes mellitus is at increased risk for which of the following fetal complications if her glycemic control is poor?
Explanation
Pre-gestational diabetes mellitus in pregnancy increases risk of congenital anomalies due to hyperglycemia during organogenesis (first 8 weeks). Poor control causes oxidative stress, disrupting cardiac, neural tube, and skeletal development. Normal fasting glucose is 70–99 mg/dL, and HbA1c target in pregnancy is <6–6.5%. Poorly controlled diabetes also increases risks of macrosomia, spontaneous abortion, polyhydramnios, and neonatal hypoglycemia.
Rationale for correct answer
3. Congenital anomalies are strongly associated with pre-gestational diabetes and poor glycemic control, particularly affecting the heart, neural tube, and musculoskeletal system. This risk is highest during early embryogenesis.
Rationale for incorrect answers
1. Fetal alcohol syndrome results from maternal alcohol use, not diabetes. It causes microcephaly, growth restriction, and facial dysmorphisms, unrelated to hyperglycemia.
2. Neonatal abstinence syndrome is caused by maternal opioid use, leading to withdrawal symptoms after birth. It is not linked to maternal diabetes.
4. Poor maternal glycemic control increases risk of respiratory distress syndrome due to delayed—not premature—lung maturation. High maternal glucose and insulin levels suppress fetal surfactant production, delaying pulmonary maturity.
Take home points
- Poor glycemic control in pre-gestational diabetes increases congenital anomaly risk.
- Common malformations involve the cardiac, neural tube, and skeletal systems.
- Alcohol and opioid use cause separate fetal syndromes, not related to diabetes.
- Hyperglycemia delays fetal lung maturation, raising risk of RDS.
What is the purpose of screening for Hepatitis B surface antigen (HBsAg) in pregnant women?
Explanation
Hepatitis B virus (HBV) infection in pregnancy is a major cause of vertical transmission to the newborn, particularly during delivery. Screening for hepatitis B surface antigen (HBsAg) at the first prenatal visit identifies women with active infection. Normal liver enzymes (ALT 7–56 U/L, AST 10–40 U/L) may be elevated in infection but screening focuses on transmission risk. Newborns of HBsAg-positive mothers must receive hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth to prevent chronic infection.
Rationale for correct answer
3. Screening for HBsAg in pregnancy is done to prevent vertical transmission. Early identification ensures immediate neonatal prophylaxis with HBV vaccine and HBIG, which reduces transmission risk from 90% to <5%.
Rationale for incorrect answers
1. Immunity to hepatitis B is determined by hepatitis B surface antibody (anti-HBs), not HBsAg. Presence of anti-HBs indicates vaccination or recovery from past infection.
2. HBsAg screening does not assess liver function. Liver function is measured with ALT, AST, bilirubin, and albumin, not HBsAg.
4. Maternal vaccination decisions are based on serology for anti-HBs and HBc antibodies, not HBsAg alone. HBsAg screening specifically targets prevention of neonatal transmission.
Take home points
- HBsAg screening at the first prenatal visit detects maternal infection.
- Vertical transmission risk is highest at delivery without prophylaxis.
- Newborns of HBsAg-positive mothers need HBV vaccine + HBIG within 12 hours.
- Anti-HBs, not HBsAg, indicates maternal immunity to hepatitis B.
What is the primary benefit of conducting the anatomy scan (Level II Ultrasound) at 18–22 weeks gestation?
Explanation
Anatomy scan (Level II ultrasound) performed at 18–22 weeks gestation evaluates fetal structural development. It provides detailed assessment of organs, skeletal structures, placental location, and amniotic fluid volume (normal AFI 8–24 cm). This scan identifies congenital anomalies such as cardiac defects, neural tube defects, and abdominal wall defects. It also confirms fetal number, gender if desired, and growth parameters. Early detection allows for counseling, prenatal interventions, or delivery planning for affected fetuses.
Rationale for correct answer
3. The primary benefit of the anatomy scan is detection of structural anomalies. Detailed imaging identifies congenital defects in the heart, brain, spine, kidneys, and limbs, allowing timely management and family counseling.
Rationale for incorrect answers
1. Fetal heart tones are typically assessed via Doppler starting at 10–12 weeks. Anatomy scan is not intended primarily for confirming heart activity.
2. Gestational diabetes screening is performed at 24–28 weeks using glucose tolerance testing. Anatomy scan does not assess maternal glucose metabolism.
4. Maternal risk for preeclampsia is assessed through blood pressure monitoring, urine protein evaluation, and maternal history. Ultrasound does not predict preeclampsia risk.
Take home points
- Anatomy scan at 18–22 weeks evaluates fetal structural development and detects congenital anomalies.
- Placental location and amniotic fluid volume are also assessed.
- Fetal heart tones and maternal conditions are evaluated through other methods.
- Early detection allows for prenatal counseling and delivery planning.
Which of the following are key components of the comprehensive history taken during the initial prenatal visit? Select all that apply.
Explanation
Comprehensive prenatal history is critical for identifying risk factors that may affect maternal or fetal health. It includes family history, past medical and surgical history, obstetric history, and social history, including substance use. Normal maternal hemoglobin is 12–16 g/dL, and hematocrit 36–46%; abnormalities in history may indicate increased risk for anemia or other complications. Dietary habits are assessed if they impact health or pregnancy outcomes, but personal preferences unrelated to medical conditions are not essential.
Rationale for correct answers
1. A detailed family history identifies genetic disorders that may affect the fetus, such as cystic fibrosis, sickle cell disease, or trisomies. This informs risk assessment and potential genetic counseling.
2. Past surgical history is important because previous abdominal or pelvic surgeries may affect pregnancy management, labor, or delivery options. Knowledge of prior procedures aids in planning safe maternal care.
4. Social history, including substance use (tobacco, alcohol, illicit drugs), is crucial to assess risks for fetal growth restriction, preterm birth, or congenital anomalies. It guides counseling and intervention.
Rationale for incorrect answers
3. Current preferences for newborn clothing are not medically relevant to prenatal risk assessment and do not impact pregnancy management.
5. Dietary restrictions unrelated to medical conditions are not essential in the initial prenatal history unless they affect nutritional adequacy or pregnancy outcomes. Focus is on medically significant nutrition needs.
Take home points
- Comprehensive prenatal history identifies maternal, fetal, and genetic risk factors.
- Family history and past surgical history guide risk assessment and delivery planning.
- Social history, including substance use, directly impacts fetal outcomes.
- Non-medically relevant preferences or dietary habits are not critical components.
Which of the following physical assessment findings are typically monitored during subsequent prenatal visits? Select all that apply.
Explanation
Routine prenatal physical assessment focuses on monitoring maternal and fetal well-being throughout pregnancy. Key components include maternal vital signs, fetal heart tones, and fundal height. Normal blood pressure is <140/90 mmHg, heart rate 60–100 bpm, respiratory rate 12–20/min, and temperature 36.5–37.5°C. Fundal height in centimeters approximates gestational age between 20–36 weeks ±2 cm. These assessments help identify complications such as preeclampsia, fetal growth restriction, or multiple gestation.
Rationale for correct answer
1. Maternal vital signs are monitored at every prenatal visit to detect hypertension, tachycardia, or fever. Abnormal readings may indicate preeclampsia, infection, or other maternal complications.
2. Fetal heart tones are assessed to ensure fetal well-being. Normal fetal heart rate ranges from 110–160 bpm, and deviations may signal fetal distress requiring further evaluation.
3. Fundal height is measured to monitor fetal growth and amniotic fluid volume. Deviations >2 cm from gestational age may indicate intrauterine growth restriction, macrosomia, or oligohydramnios/polyhydramnios.
Rationale for incorrect answers
4. A detailed neurological examination of the mother is not routinely performed unless there are specific symptoms such as headaches, vision changes, or neurological deficits. It is not part of standard prenatal visits.
5. Extensive gynecological examination is not required at every visit. Pelvic exams are typically done at the initial visit and selectively based on risk factors or complications. Routine subsequent visits focus on maternal and fetal assessments.
Take home points
- Maternal vital signs monitor for hypertension, infection, and maternal complications.
- Fetal heart tones ensure fetal well-being and detect distress early.
- Fundal height tracks fetal growth and amniotic fluid status.
- Neurological and extensive gynecological exams are done only when indicated.
Which of the following laboratory tests are routinely performed at the initial prenatal visit? Select all that apply.
Explanation
Initial prenatal laboratory testing is essential to assess maternal hematologic, immunologic, and infectious status to optimize maternal and fetal outcomes. Common tests include CBC to detect anemia (normal hemoglobin 12–16 g/dL, hematocrit 36–46%), blood type and Rh factor to identify alloimmunization risk, and rubella titer to determine immunity (positive ≥1:10). These tests help guide prophylaxis, vaccination, and management decisions early in pregnancy. Other tests may include syphilis, HIV, hepatitis B, and urine studies depending on epidemiology and risk factors.
Rationale for correct answer
1. CBC is routinely performed at the initial prenatal visit to detect anemia, leukopenia, or thrombocytopenia. Maternal hemoglobin below 11 g/dL in the first trimester indicates iron-deficiency anemia, guiding supplementation.
2. Blood type and Rh factor identify potential Rh incompatibility. Rh-negative mothers require RhoGAM prophylaxis to prevent hemolytic disease of the newborn in future pregnancies.
4. Rubella titer determines immunity. Nonimmune women (titer <1:10) are counseled to avoid pregnancy until vaccination, as rubella infection can cause congenital rubella syndrome.
Rationale for incorrect answers
3. Hepatitis C antibody screening is not routinely performed at the initial prenatal visit unless the patient has specific risk factors such as intravenous drug use, history of blood transfusions before 1992, or known HCV exposure. Universal screening is not standard practice.
5. Thyroid function tests are not routinely done in all pregnant women. Screening is indicated only for those with a history of thyroid disease, symptoms suggestive of hypo- or hyperthyroidism, or high-risk factors such as autoimmune disease or previous pregnancy complications.
Take home points
- CBC, blood type/Rh, and rubella titer are standard first-trimester labs.
- Anemia is defined as hemoglobin <11 g/dL in the first trimester.
- Rh-negative mothers require prophylaxis to prevent hemolytic disease of the newborn.
- Hepatitis C and thyroid function tests are performed selectively based on risk or history.
Exams on Maternal Risk Factors
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
- Objective
- Introduction
- Maternal Age-related Risks
- Practice Exercise 1
- Preexisting Medical Conditions
- Practice Exercise 2
- Infectious Disease Risks
- Practice Exercise 3
- Substance Use And Environmental Exposures
- Practice Exercise 4
- Psychosocial And Socioeconomic Factors
- Obstetric And Fetal Complications
- Practice Exercise 5
- Nutritional Deficiencies And Maternal Obesity
- Practice Exercise 6
- Summary
- Comprehensive Questions
Notes Highlighting is available once you sign in. Login Here.
Objective
- Analyze the physiological and clinical implications of adolescent pregnancy and advanced maternal age on maternal and fetal health, identifying associated risks such as preterm birth, chromosomal abnormalities, and obstetric complications.
- Evaluate the impact of chronic maternal conditions, including diabetes mellitus, hypertension, and thyroid disorders, on pregnancy outcomes, emphasizing pathophysiology, fetal risks, and nursing interventions.
- Describe the epidemiology, transmission, and congenital effects of TORCH infections, sexually transmitted infections, and Group B Streptococcus, highlighting preventive measures and neonatal implications.
- Assess the teratogenic effects of tobacco, alcohol, illicit drugs, and environmental toxins on fetal development, detailing nursing strategies for risk reduction and patient education.
- Examine the influence of intimate partner violence, depression, and socioeconomic barriers on prenatal care adherence and pregnancy outcomes, focusing on screening and intervention techniques.
- Identify the pathophysiology and clinical management of obstetric complications such as multiple gestation, placental abnormalities, and intrauterine growth restriction, emphasizing fetal surveillance and maternal care.
- Explore the consequences of folic acid and iron deficiencies, as well as maternal obesity, on maternal and fetal health, outlining nutritional counseling and monitoring strategies.
Introduction
Maternal-newborn nursing requires a comprehensive understanding of risk factors that adversely affect pregnancy outcomes to ensure optimal maternal and fetal health. These risk factors encompass a broad spectrum of physiological, environmental, psychosocial, and socioeconomic elements that can significantly influence the course of pregnancy, labor, and neonatal development. Nurses play a pivotal role in identifying, managing, and mitigating these risks through evidence-based assessments, interventions, and patient education. This set of notes provides an in-depth exploration of critical risk factors, including maternal age, preexisting medical conditions, infectious diseases, substance use, environmental exposures, psychosocial stressors, obstetric complications, and nutritional deficiencies. By addressing these factors systematically, nurses can enhance prenatal care, reduce adverse outcomes, and promote healthy pregnancies. Each section includes detailed scientific explanations, practical nursing insights, and practice questions to facilitate learning and application in clinical settings. The goal is to prepare nursing students to recognize high-risk pregnancies, implement appropriate interventions, and educate patients effectively to optimize maternal and neonatal outcomes.
Maternal Age-related Risks
Maternal age is a critical determinant of pregnancy outcomes, with both extremes—adolescence and advanced maternal age—posing unique challenges. These age-related risks impact maternal physiology, fetal development, and obstetric outcomes, necessitating tailored nursing assessments and interventions to mitigate complications.
3.1 Adolescent Pregnancy
Adolescent pregnancy, defined as pregnancy in individuals aged 10 to 19 years, is associated with increased maternal and fetal risks due to physiological immaturity, socioeconomic challenges, and limited access to prenatal care. Adolescents often face higher rates of preterm birth, low birth weight, and hypertensive disorders, compounded by psychosocial stressors such as inadequate support systems.
- Physiological Risks: The adolescent pelvis may not be fully developed, increasing the risk of cephalopelvic disproportion (CPD), which can lead to prolonged labor or the need for cesarean delivery. Immature uterine vasculature may contribute to inadequate placental perfusion, resulting in intrauterine growth restriction (IUGR).
- Adolescents are at a higher risk for hypertensive disorders, including preeclampsia, due to underdeveloped vascular responses.
- Nutritional deficiencies are prevalent due to poor dietary habits and competing growth demands, increasing the risk of anemia and preterm labor.
- Psychosocial Risks: Adolescents often face social stigma, limited financial resources, and inadequate access to healthcare, leading to delayed or inconsistent prenatal care.
- Higher rates of intimate partner violence (IPV) and depression are reported, which can exacerbate poor pregnancy outcomes.
- Fetal and Neonatal Risks: Infants born to adolescent mothers have a higher incidence of low birth weight (LBW) and prematurity, contributing to increased neonatal morbidity and mortality.

- There is an elevated risk of sudden infant death syndrome (SIDS) due to factors such as maternal smoking or unsafe sleep practices.
Nursing Insights
- Conduct a thorough psychosocial assessment during the initial prenatal visit to identify barriers to care, such as lack of transportation or family support.
- Educate adolescent patients on the importance of consistent prenatal care to monitor for complications like preeclampsia and IUGR.
- Assess nutritional status and provide counseling on a balanced diet rich in iron, calcium, and folic acid to support both maternal and fetal growth.
- Screen for IPV and depression using validated tools like the Edinburgh Postnatal Depression Scale to ensure early intervention.
- Collaborate with social workers to connect adolescents with community resources, such as Medicaid or teen parenting programs.
3.2 Advanced Maternal Age
Advanced maternal age (AMA), defined as pregnancy in women aged 35 years and older, is associated with increased risks of chromosomal abnormalities, obstetric complications, and maternal comorbidities. The decline in oocyte quality and physiological reserve contributes to adverse outcomes, requiring vigilant monitoring.
- Chromosomal Abnormalities: Aging oocytes are prone to nondisjunction, leading to an increased incidence of aneuploidies such as Down syndrome (trisomy 21), trisomy 18, and trisomy 13.
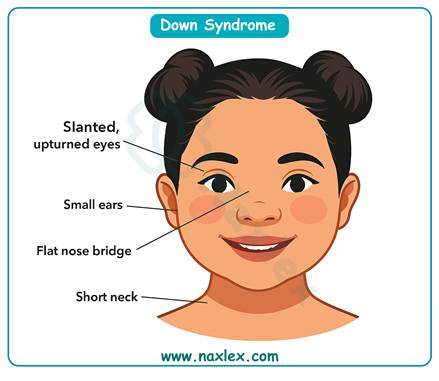
-
- The risk of Down syndrome rises significantly, from 1 in 1,250 at age 25 to 1 in 100 at age 40.
- Obstetric Complications: Women with AMA have a higher prevalence of gestational diabetes mellitus (GDM), preeclampsia, and placenta previa due to vascular changes and reduced uterine elasticity.
- Increased rates of cesarean delivery are observed due to prolonged labor, fetal distress, or maternal medical conditions.
- Maternal Comorbidities: AMA is associated with a higher incidence of chronic conditions such as hypertension, diabetes, and obesity, which exacerbate pregnancy risks.
- Preexisting medical conditions may worsen, requiring careful management to prevent adverse outcomes.
- Fetal and Neonatal Risks: Fetuses of mothers with AMA are at risk for preterm birth, stillbirth, and congenital anomalies due to placental insufficiency or maternal health issues.
- Macrosomia may occur in the presence of GDM, increasing the risk of shoulder dystocia during delivery.
Nursing Insights
- Offer first-trimester screening, including nuchal translucency ultrasound and maternal serum markers, to assess for aneuploidy risks.
- Monitor for signs of GDM and preeclampsia, including blood glucose levels and blood pressure, at each prenatal visit.
- Educate patients on the importance of genetic counseling to discuss risks and options for invasive testing, such as amniocentesis or chorionic villus sampling (CVS).
- Assess fundal height and fetal heart rate to detect early signs of IUGR or placental dysfunction.
- Provide anticipatory guidance on the potential need for cesarean delivery and the associated recovery process.
Preexisting Medical Conditions
Preexisting medical conditions significantly influence pregnancy outcomes, requiring meticulous management to prevent maternal and fetal complications. Conditions such as diabetes mellitus, hypertension, and thyroid disorders alter maternal physiology and necessitate specialized nursing care.
5.1 Diabetes Mellitus
Diabetes mellitus, whether pregestational or gestational, poses significant risks to maternal and fetal health due to altered glucose metabolism and vascular complications. Poor glycemic control is associated with congenital anomalies, macrosomia, and neonatal hypoglycemia.
- Pathophysiology: Hyperglycemia in early pregnancy disrupts organogenesis, increasing the risk of congenital anomalies, particularly cardiac and neural tube defects.
- Poorly controlled diabetes leads to fetal hyperglycemia, stimulating insulin production and resulting in macrosomia (birth weight >4,000 g).
- Maternal Risks: Women with diabetes are at increased risk for preeclampsia, polyhydramnios, and cesarean delivery due to fetal macrosomia or labor complications.
- Long-term, women with gestational diabetes mellitus (GDM) face a higher risk of developing type 2 diabetes mellitus later in life.
- Fetal and Neonatal Risks: Macrosomia increases the risk of shoulder dystocia, birth trauma, and neonatal hypoglycemia due to sudden cessation of maternal glucose supply at birth.
- Congenital anomalies, such as caudal regression syndrome, are more prevalent in pregestational diabetes.
- Management: Glycemic control is achieved through diet, exercise, insulin therapy, or oral hypoglycemic agents as prescribed. Regular monitoring of blood glucose levels is critical.
- The one-hour glucose tolerance test (GTT) is performed at 24–28 weeks gestation to screen for GDM.
Nursing Insights
- Monitor blood glucose levels at each prenatal visit and educate patients on self-monitoring techniques using glucometers.
- Provide nutritional counseling, emphasizing a balanced diet with controlled carbohydrate intake to maintain euglycemia.
- Assess for signs of preeclampsia, such as hypertension and proteinuria, in patients with diabetes.
- Educate patients on the importance of fetal movement monitoring to detect potential distress in macrosomic fetuses.
- Prepare for potential neonatal complications by ensuring neonatal intensive care unit (NICU) availability at delivery.
5.2 Hypertension
Chronic hypertension and gestational hypertensive disorders, including preeclampsia and eclampsia, are significant contributors to maternal and fetal morbidity. These conditions impair placental perfusion and increase the risk of adverse outcomes.
- Pathophysiology: Chronic hypertension, defined as blood pressure ≥140/90 mmHg before pregnancy or before 20 weeks gestation, causes vascular remodeling that reduces placental blood flow.
- Preeclampsia, characterized by hypertension and proteinuria after 20 weeks gestation, results from endothelial dysfunction and systemic vasoconstriction.
- Maternal Risks: Women with chronic hypertension are at increased risk for preeclampsia, placental abruption, and stroke.
- Eclampsia, a severe complication of preeclampsia, presents with seizures and requires immediate intervention with magnesium sulfate.
- Fetal Risks: Reduced placental perfusion leads to IUGR, preterm birth, and stillbirth.
- Fetal hypoxia may occur due to impaired uteroplacental blood flow, necessitating frequent fetal surveillance.
- Management: Antihypertensive medications, such as methyldopa or labetalol, are used to manage blood pressure. Low-dose aspirin may be prescribed to reduce preeclampsia risk.
- Fetal well-being is assessed through non-stress tests (NSTs) and biophysical profiles (BPPs).
Nursing Insights
- Monitor blood pressure at each prenatal visit and educate patients on home blood pressure monitoring.
- Assess for symptoms of preeclampsia, including severe headaches, epigastric pain, and visual disturbances, and report immediately.
- Administer magnesium sulfate as prescribed for seizure prophylaxis in preeclampsia or eclampsia, monitoring for toxicity (e.g., respiratory depression, loss of deep tendon reflexes).
- Educate patients on the importance of bed rest or activity restriction if prescribed for hypertensive disorders.
- Perform NSTs and BPPs to evaluate fetal well-being, ensuring a reactive NST (two accelerations in 20 minutes) indicates normal fetal status.
5.3 Thyroid Disorders
Thyroid disorders, including hypothyroidism and hyperthyroidism, affect maternal metabolism and fetal neurodevelopment. Untreated conditions can lead to significant complications, necessitating careful monitoring and management.

- Hypothyroidism: Characterized by insufficient thyroid hormone production, hypothyroidism is associated with increased risks of miscarriage, preeclampsia, and preterm birth.
- Fetal neurodevelopmental issues, including cognitive delays, may occur due to inadequate thyroid hormone transfer across the placenta.
- Hyperthyroidism: Excessive thyroid hormone production, often due to Graves’ disease, increases the risk of maternal heart failure, thyroid storm, and preterm labor.
- Fetal risks include IUGR, congenital anomalies, and neonatal thyrotoxicosis due to transplacental passage of thyroid-stimulating antibodies.
- Management: Levothyroxine is used to treat hypothyroidism, with thyroid-stimulating hormone (TSH) levels monitored to ensure adequacy of replacement therapy.
- Antithyroid medications, such as propylthiouracil (PTU), are used in hyperthyroidism, with careful monitoring to avoid fetal hypothyroidism.
- Screening: Thyroid function tests (TSH, free T4) are indicated in women with a history of thyroid disease or symptoms suggestive of dysfunction.
Nursing Insights
- Monitor thyroid function test results and ensure patients adhere to prescribed thyroid medications.
- Educate patients on symptoms of thyroid dysfunction, such as fatigue, weight changes, or palpitations, and the importance of reporting them.
- Assess fetal growth through ultrasound to detect IUGR in mothers with thyroid disorders.
- Collaborate with endocrinologists to optimize thyroid hormone levels before and during pregnancy.
- Educate patients on the importance of avoiding iodine excess, which can exacerbate hyperthyroidism.
Infectious Disease Risks
Infectious diseases during pregnancy pose significant risks to maternal and fetal health, potentially leading to congenital anomalies, preterm birth, and neonatal sepsis. The TORCH infections, other sexually transmitted infections (STIs), and Group B Streptococcus (GBS) are critical pathogens that require vigilant screening, prevention, and management to optimize pregnancy outcomes.
7.1 TORCH Infections
TORCH infections, an acronym for Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes simplex virus (HSV), are associated with severe congenital anomalies when contracted during pregnancy, particularly in the first trimester. These infections can cross the placenta, leading to significant fetal morbidity.
- Toxoplasmosis: Caused by the protozoan Toxoplasma gondii, toxoplasmosis is transmitted through undercooked meat, contaminated water, or contact with infected cat feces.
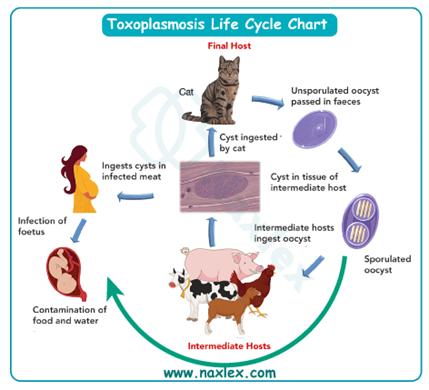
-
- Maternal Effects: Often asymptomatic or presenting with flu-like symptoms, including fever and lymphadenopathy.
- Fetal Effects: Congenital toxoplasmosis may result in the classic triad of chorioretinitis, hydrocephalus, and intracranial calcifications, leading to vision loss and neurodevelopmental delays.
- Management: Spiramycin is used to reduce transplacental transmission, with pyrimethamine and sulfadiazine reserved for confirmed fetal infection.
- Syphilis: Caused by Treponema pallidum, syphilis is a sexually transmitted infection that can lead to congenital syphilis if untreated.
- Maternal Effects: Primary syphilis presents with a chancre, while secondary syphilis may cause rash, fever, and lymphadenopathy.
- Fetal Effects: Congenital syphilis can cause hepatosplenomegaly, skeletal abnormalities (e.g., saber shins), and neurosyphilis, leading to developmental delays.
- Management: Penicillin G is the treatment of choice, with desensitization for penicillin-allergic patients.
- Rubella: A viral infection transmitted via respiratory droplets, rubella is particularly teratogenic in the first trimester.
- Maternal Effects: Mild fever, rash, and arthralgia are common, though many cases are asymptomatic.
- Fetal Effects: Congenital rubella syndrome (CRS) includes cardiac defects (e.g., patent ductus arteriosus), cataracts, and sensorineural deafness.
- Management: Prevention through MMR vaccination before pregnancy is critical, as no specific antiviral treatment exists.
- Cytomegalovirus (CMV): The most common congenital infection, CMV is transmitted through bodily fluids (e.g., saliva, urine).
- Maternal Effects: Often asymptomatic or presenting with mononucleosis-like symptoms.
- Fetal Effects: Congenital CMV may cause microcephaly, sensorineural hearing loss, and developmental delays.
- Management: Antiviral therapy (e.g., valganciclovir) may be considered for severe cases, but prevention through hygiene practices is key.
- Herpes Simplex Virus (HSV): Transmitted through sexual contact or during delivery, HSV poses risks primarily during the third trimester or labor.
- Maternal Effects: Genital lesions, fever, and lymphadenopathy are common during primary infection.
- Fetal Effects: Neonatal HSV can cause disseminated infection, encephalitis, or localized skin/eye/mouth disease, with high mortality if untreated.
- Management: Acyclovir is used for maternal treatment, with cesarean delivery recommended for active genital lesions at labor.
Nursing Insights
- Educate pregnant patients on preventing toxoplasmosis by avoiding undercooked meat, washing produce thoroughly, and avoiding contact with cat litter.
- Screen all pregnant women for syphilis using the Venereal Disease Research Laboratory (VDRL) or Rapid Plasma Reagin (RPR) test at the initial prenatal visit.
- Verify rubella immunity through a rubella titer and counsel non-immune patients to avoid exposure to infected individuals.
- Teach hygiene practices to prevent CMV transmission, such as handwashing after contact with young children’s bodily fluids.
- Assess for active HSV lesions during labor and prepare for cesarean delivery if lesions are present to prevent neonatal transmission.
7.2 Other Sexually Transmitted Infections
Other STIs, including chlamydia, gonorrhea, and human immunodeficiency virus (HIV), can adversely affect pregnancy outcomes, leading to preterm labor, neonatal infections, and long-term sequelae if untreated.
- Chlamydia: Caused by Chlamydia trachomatis, chlamydia is a common STI that is often asymptomatic in women.
- Maternal Effects: Can cause cervicitis, pelvic inflammatory disease (PID), and increased risk of preterm labor.
- Fetal/Neonatal Effects: Neonatal conjunctivitis and pneumonia are common complications of perinatal transmission.
- Management: Azithromycin or erythromycin is used for treatment, with screening recommended at the first prenatal visit and in the third trimester for high-risk patients.
- Gonorrhea: Caused by Neisseria gonorrhoeae, gonorrhea increases the risk of preterm birth and premature rupture of membranes (PROM).
- Maternal Effects: May present with mucopurulent discharge or be asymptomatic.
- Fetal/Neonatal Effects: Neonatal ophthalmia neonatorum can lead to blindness if untreated.
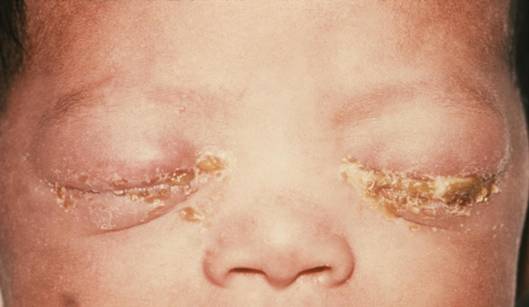
- Management: Ceftriaxone is the treatment of choice, with dual therapy for chlamydia co-infection due to high concurrence rates.
- HIV: Transmitted through sexual contact, blood, or perinatal routes, HIV poses significant risks without intervention.
- Maternal Effects: Advanced HIV may lead to opportunistic infections, impacting pregnancy outcomes.
- Fetal/Neonatal Effects: Vertical transmission can occur in utero, during delivery, or via breastfeeding, leading to pediatric HIV infection.
- Management: Antiretroviral therapy (ART) is used to suppress viral load, with cesarean delivery recommended for women with high viral loads (>1,000 copies/mL).
Nursing Insights
- Perform routine STI screening at the initial prenatal visit, including tests for chlamydia, gonorrhea, and HIV, to ensure early detection and treatment.
- Educate patients on safe sexual practices, including condom use, to prevent STI transmission during pregnancy.
- Administer erythromycin eye ointment to newborns to prevent ophthalmia neonatorum caused by gonorrhea or chlamydia.
- Collaborate with infectious disease specialists to manage HIV-positive pregnant patients and ensure adherence to ART.
- Monitor for signs of preterm labor in patients with untreated STIs and report abnormal findings promptly.
7.3 Group B Streptococcus
Group B Streptococcus (GBS) is a gram-positive bacterium commonly colonizing the maternal gastrointestinal and genitourinary tracts, posing risks to the neonate during delivery.
- Epidemiology: Approximately 10–30% of pregnant women are colonized with GBS, with higher rates in certain populations.
- Maternal Effects: GBS is usually asymptomatic in pregnant women but can cause urinary tract infections, chorioamnionitis, or postpartum endometritis.
- Fetal/Neonatal Effects: Neonatal GBS infection can manifest as early-onset (within 7 days) or late-onset (7–90 days) disease, leading to sepsis, pneumonia, or meningitis.
- Early-onset GBS disease is associated with maternal colonization and intrapartum transmission.
- Late-onset disease may result from community or hospital-acquired transmission.
- Screening and Management: Universal screening is performed at 35–37 weeks gestation using rectovaginal cultures.
- Intrapartum antibiotic prophylaxis (IAP) with penicillin or ampicillin is administered to GBS-positive women or those with risk factors (e.g., preterm labor, PROM >18 hours, or maternal fever).
- Clindamycin or vancomycin is used for penicillin-allergic patients with confirmed GBS sensitivity.
Nursing Insights
- Ensure GBS screening is completed at 35–37 weeks gestation and document results in the patient’s chart for intrapartum management.
- Administer IAP as prescribed, typically penicillin every 4 hours during labor, ensuring at least one dose is given 4 hours before delivery to reduce neonatal transmission.
- Monitor neonates born to GBS-positive mothers for signs of sepsis, such as respiratory distress, lethargy, or poor feeding, in the first 24–48 hours.
- Educate patients on the importance of reporting fever, prolonged rupture of membranes, or preterm labor, as these increase GBS transmission risk.
- Verify neonatal administration of post-delivery observation protocols for GBS-exposed infants, including vital signs and clinical assessment.
Substance Use And Environmental Exposures
Substance use and environmental exposures during pregnancy are significant risk factors for adverse maternal and fetal outcomes. Tobacco, alcohol, illicit drugs, and environmental toxins act as teratogens, disrupting fetal development and increasing the risk of complications.
9.1 Tobacco and Smoking
Maternal smoking and exposure to secondhand smoke are associated with a range of adverse pregnancy outcomes, primarily due to the toxic effects of nicotine, carbon monoxide, and other chemicals on placental function and fetal oxygenation.
- Maternal Effects: Smoking increases the risk of placental abruption, preterm labor, and spontaneous abortion due to vascular constriction and reduced oxygen delivery.
- Chronic smoking may exacerbate maternal respiratory conditions, such as asthma, complicating pregnancy management.
- Fetal/Neonatal Effects: Intrauterine growth restriction (IUGR) is a common consequence due to impaired placental perfusion, leading to low birth weight.
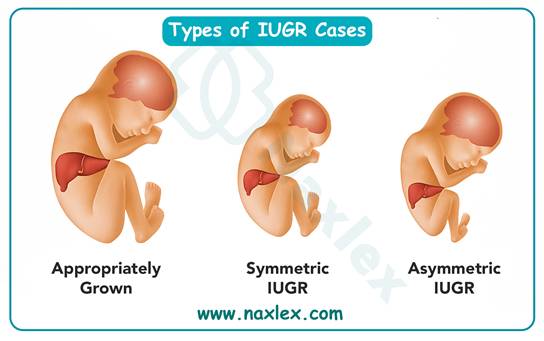
-
- Preterm birth and sudden infant death syndrome (SIDS) are significantly associated with maternal smoking, with SIDS risk increasing due to altered neonatal respiratory control.
- Congenital anomalies, such as cleft lip/palate, may occur due to the teratogenic effects of tobacco compounds.
- Management: Smoking cessation programs, including counseling and nicotine replacement therapy (if deemed safe), are critical interventions.
- Nurses should assess smoking status at each prenatal visit and provide tailored cessation support.
Nursing Insights
- Use the 5 A’s framework (Ask, Advise, Assess, Assist, Arrange) to address smoking cessation at every prenatal visit.
- Educate patients on the risks of secondhand smoke exposure and encourage smoke-free environments.
- Monitor fetal growth via ultrasound to detect IUGR in patients with a history of smoking.
- Collaborate with behavioral health specialists to provide counseling for smoking cessation.
- Assess neonatal respiratory status post-delivery in infants exposed to maternal smoking, watching for signs of distress.
9.2 Alcohol and Illicit Drugs
Alcohol and illicit drug use during pregnancy are highly teratogenic, leading to a spectrum of congenital anomalies and developmental disorders. There is no safe level of alcohol consumption during pregnancy.
- Alcohol: Ethanol crosses the placenta, causing fetal alcohol spectrum disorders (FASD), including fetal alcohol syndrome (FAS).
- Fetal Effects: FAS is characterized by facial dysmorphia (e.g., smooth philtrum, thin upper lip), growth restriction, and neurodevelopmental deficits.
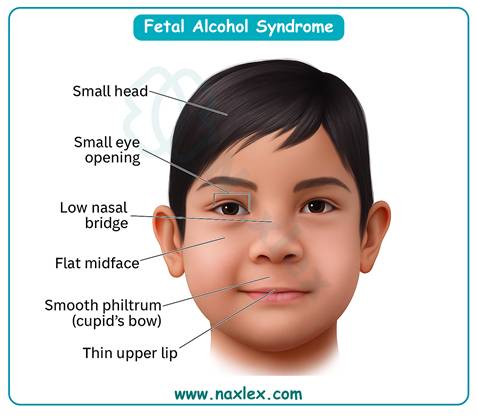
- Management: Complete abstinence is recommended, with counseling and support groups (e.g., Alcoholics Anonymous) for cessation.
- Opioids: Maternal opioid use, including prescription misuse and illicit use (e.g., heroin), leads to neonatal abstinence syndrome (NAS).
- Neonatal Effects: NAS presents with tremors, irritability, poor feeding, and seizures due to withdrawal post-delivery.
- Management: Methadone or buprenorphine maintenance therapy is used for opioid-dependent pregnant women to stabilize maternal health and reduce fetal risks.
- Cocaine: Cocaine use causes vasoconstriction, leading to placental abruption, preterm labor, and IUGR.
- Fetal Effects: Neurobehavioral deficits and congenital anomalies (e.g., limb defects) may occur.
- Management: Multidisciplinary care, including substance abuse counseling and social services, is essential.
- Marijuana: Though less studied, marijuana use is associated with low birth weight and potential neurodevelopmental issues.
- Management: Patients should be counseled to avoid marijuana due to limited safety data.
Nursing Insights
- Screen for substance use using validated tools (e.g., T-ACE or 4Ps) at the initial prenatal visit and throughout pregnancy.
- Educate patients that no amount of alcohol is safe during pregnancy, emphasizing the risk of FASD.
- Monitor neonates for signs of NAS, including tremors and poor feeding, and ensure NICU availability for affected infants.
- Refer patients to substance abuse treatment programs and social services to address underlying addiction issues.
- Assess for placental abruption in patients using cocaine, watching for vaginal bleeding or abdominal pain.
9.3 Environmental Toxins
Environmental exposures to toxins such as heavy metals, air pollution, and radiation can disrupt fetal development, leading to miscarriage, congenital anomalies, and developmental delays.
- Heavy Metals (Lead, Mercury): Lead exposure, often from contaminated water or paint, increases the risk of miscarriage, IUGR, and neurodevelopmental delays.
- Mercury, found in certain fish (e.g., tuna, swordfish), can cause neurological damage and developmental delays in the fetus.
- Management: Patients should avoid high-mercury fish and be screened for lead exposure in high-risk environments.
- Air Pollution: Particulate matter and pollutants are associated with preterm birth, low birth weight, and congenital heart defects.
- Management: Advise patients to avoid high-pollution areas and use air purifiers in urban settings.
- Radiation: Ionizing radiation (e.g., from medical imaging) poses risks of congenital anomalies and growth restriction, particularly in the first trimester.
- Management: Limit unnecessary imaging and use shielding during essential procedures.
Nursing Insights
- Educate patients to avoid high-mercury fish (e.g., tuna, mackerel) and consume low-mercury options (e.g., salmon, tilapia).
- Screen for lead exposure in patients living in older homes or high-risk areas, coordinating with public health resources for testing.
- Advise patients to minimize outdoor activities during high air pollution days and monitor air quality indexes.
- Ensure informed consent for any radiographic procedures, explaining risks and benefits to pregnant patients.
- Collaborate with environmental health specialists to address toxin exposure risks in the patient’s community.
Psychosocial And Socioeconomic Factors
Psychosocial and socioeconomic factors significantly influence pregnancy outcomes by affecting maternal health, prenatal care adherence, and fetal well-being. Intimate partner violence (IPV), depression, socioeconomic barriers, and food insecurity create complex challenges that require comprehensive nursing assessments and interventions to mitigate adverse effects.
11.1 Intimate Partner Violence and Depression
Intimate partner violence and depression are critical psychosocial risk factors that increase the likelihood of adverse pregnancy outcomes, including preterm birth, low birth weight, and maternal morbidity.
- Intimate Partner Violence (IPV): IPV encompasses physical, emotional, or sexual abuse by a partner, with increased prevalence during pregnancy due to heightened stress and power dynamics.
- Maternal Effects: IPV is associated with physical injuries, chronic stress, and increased risk of miscarriage, preterm labor, and placental abruption.
- Fetal/Neonatal Effects: Fetal growth restriction, preterm birth, and neonatal stress responses are linked to maternal stress from IPV.
- Screening and Management: Routine screening using validated tools, such as the Abuse Assessment Screen (AAS), is essential during prenatal visits. Interventions include safety planning, referrals to domestic violence shelters, and counseling.
- Depression: Perinatal depression, encompassing antenatal and postnatal depression, affects approximately 10–20% of pregnant women and is characterized by persistent sadness, anxiety, or loss of interest.
- Maternal Effects: Depression is associated with poor prenatal care adherence, substance use, and increased risk of postpartum depression.
- Fetal/Neonatal Effects: Maternal depression may lead to preterm birth, low birth weight, and impaired neonatal neurobehavioral development due to altered maternal cortisol levels.
- Management: Screening with tools like the Edinburgh Postnatal Depression Scale (EPDS) is recommended. Treatment includes psychotherapy (e.g., cognitive-behavioral therapy) and, if necessary, selective serotonin reuptake inhibitors (SSRIs) deemed safe for pregnancy.
Nursing Insights
- Conduct private, sensitive screenings for IPV during prenatal visits, ensuring a safe environment free from the presence of partners or family members.
- Use the EPDS to screen for depression at least once per trimester and postpartum, referring patients with scores ≥10 to mental health services.
- Develop individualized safety plans for patients experiencing IPV, including contact information for local shelters and hotlines.
- Educate patients on the impact of stress and depression on fetal development, emphasizing the importance of mental health care.
- Collaborate with social workers and mental health professionals to provide comprehensive support for patients with IPV or depression.
11.2 Socioeconomic Barriers and Food Insecurity
Socioeconomic barriers and food insecurity exacerbate disparities in maternal and fetal health outcomes by limiting access to healthcare, nutrition, and stable living conditions.
- Socioeconomic Barriers: Low income, lack of health insurance, and limited access to transportation or healthcare facilities contribute to delayed or inadequate prenatal care.
- Maternal Effects: Inconsistent prenatal care increases the risk of undetected complications, such as preeclampsia or gestational diabetes mellitus (GDM).
- Fetal/Neonatal Effects: Inadequate prenatal care is associated with preterm birth, low birth weight, and increased neonatal morbidity.
- Management: Nurses should connect patients with community resources, such as Medicaid, transportation assistance, and prenatal care programs.
- Food Insecurity: Defined as limited or uncertain access to adequate food, food insecurity affects maternal nutrition and fetal growth.
- Maternal Effects: Food insecurity is linked to nutritional deficiencies, anemia, and increased stress, contributing to poor pregnancy outcomes.
- Fetal/Neonatal Effects: Poor fetal growth, low birth weight, and congenital anomalies are associated with maternal malnutrition.
- Management: Nutritional counseling, referrals to food assistance programs (e.g., WIC), and supplementation with prenatal vitamins are critical interventions.
Nursing Insights
- Assess socioeconomic status during the initial prenatal visit, including income, insurance, and access to transportation, to identify barriers to care.
- Refer patients with food insecurity to the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) to ensure adequate nutrition.
- Educate patients on low-cost, nutrient-dense food options to optimize maternal and fetal health.
- Coordinate with case managers to facilitate access to healthcare services for patients with transportation or financial barriers.
- Monitor fetal growth through fundal height measurements and ultrasounds in patients with food insecurity to detect early signs of growth restriction.
Obstetric And Fetal Complications
Obstetric and fetal complications, including multiple gestation, placental abnormalities, and intrauterine growth restriction, significantly impact maternal and neonatal outcomes, requiring vigilant monitoring and specialized care.
12.1 Multiple Gestation
Multiple gestation, such as twin or higher-order pregnancies, increases the risk of maternal and fetal complications due to increased physiological demands and placental sharing.
- Maternal Effects: Multiple gestation is associated with a higher incidence of gestational hypertension, preeclampsia, gestational diabetes, and postpartum hemorrhage due to uterine overdistension.
- Cesarean delivery rates are elevated due to malpresentation or fetal distress.
- Fetal/Neonatal Effects: Preterm birth is the most common complication, with 50–60% of twins delivering before 37 weeks gestation.
- Twin-to-twin transfusion syndrome (TTTS) may occur in monochorionic twins, leading to unequal blood flow and growth disparities.
- Low birth weight and neonatal intensive care unit (NICU) admissions are frequent due to prematurity.
- Management: Increased prenatal visits, serial ultrasounds to monitor fetal growth, and non-stress tests (NSTs) are essential. Tocolytics may be used to delay preterm labor, and corticosteroids (e.g., betamethasone) are administered for fetal lung maturity.
Nursing Insights
- Monitor maternal vital signs and symptoms of preeclampsia (e.g., headaches, epigastric pain) at each prenatal visit due to increased risk in multiple gestation.
- Educate patients on signs of preterm labor, such as regular contractions or rupture of membranes, and the need to report them immediately.
- Perform frequent fetal surveillance, including NSTs and biophysical profiles (BPPs), to assess fetal well-being in multiple gestation pregnancies.
- Prepare patients for the possibility of cesarean delivery and discuss postpartum recovery needs, including support for multiple infants.
- Coordinate with neonatology to ensure NICU availability for preterm or low-birth-weight infants.
12.2 Placental Abnormalities
Placental abnormalities, including placenta previa and placental abruption, are serious obstetric complications that can lead to maternal hemorrhage and fetal hypoxia.
- Placenta Previa: Occurs when the placenta partially or completely covers the cervical os, leading to painless vaginal bleeding, typically in the third trimester.
- Maternal Effects: Severe hemorrhage may necessitate cesarean delivery and increase the risk of postpartum hemorrhage.
- Fetal/Neonatal Effects: Fetal hypoxia and preterm birth are common due to bleeding or premature delivery.
- Management: Ultrasound confirms diagnosis, with expectant management for stable cases and cesarean delivery for active bleeding or labor.
- Placental Abruption: The premature separation of the placenta from the uterine wall, often presenting with painful vaginal bleeding and abdominal pain.
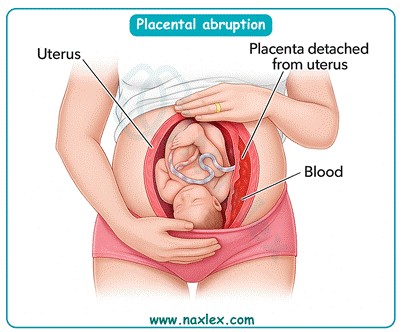
- Maternal Effects: Abruption increases the risk of hemorrhagic shock, disseminated intravascular coagulation (DIC), and maternal mortality.
- Fetal/Neonatal Effects: Fetal hypoxia, preterm birth, and stillbirth are significant risks due to reduced placental perfusion.
- Management: Immediate stabilization, fetal monitoring, and emergency cesarean delivery may be required for severe cases.
Nursing Insights
- Monitor for vaginal bleeding and abdominal pain in patients with suspected placental abnormalities, reporting findings immediately.
- Educate patients with placenta previa to avoid activities that may trigger bleeding, such as intercourse or heavy lifting.
- Perform continuous fetal heart rate monitoring in cases of placental abruption to detect signs of fetal distress.
- Prepare for rapid intervention, including blood transfusions or emergency cesarean delivery, in patients with severe hemorrhage.
- Assess postpartum patients for signs of hemorrhage or infection following placental abnormalities.
12.3 Intrauterine Growth Restriction
Intrauterine growth restriction (IUGR) is defined as fetal growth below the 10th percentile for gestational age, often resulting from placental insufficiency or maternal risk factors.
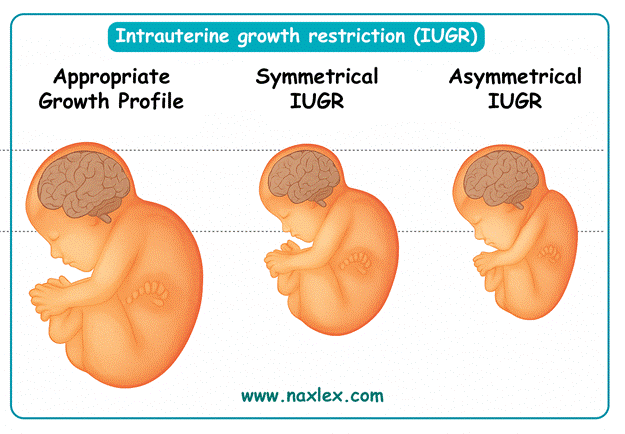
- Etiology: IUGR is associated with maternal hypertension, smoking, substance use, malnutrition, and placental abnormalities.
- Symmetrical IUGR (affecting all fetal parameters) suggests early insults, such as chromosomal abnormalities, while asymmetrical IUGR (sparing head growth) indicates late-onset issues, such as placental insufficiency.
- Maternal Effects: IUGR increases the risk of preterm labor and cesarean delivery due to fetal distress.
- Fetal/Neonatal Effects: IUGR is associated with fetal hypoxia, neonatal hypothermia, hypoglycemia, and long-term neurodevelopmental delays.
- Management: Serial ultrasounds to monitor fetal growth, Doppler studies to assess umbilical artery flow, and NSTs/BPPs are used to evaluate fetal well-being. Early delivery may be indicated for severe cases.
Nursing Insights
- Measure fundal height at each prenatal visit to detect deviations suggestive of IUGR, referring for ultrasound confirmation if needed.
- Educate patients on smoking cessation and optimal nutrition to reduce IUGR risk factors.
- Monitor fetal heart rate and movement patterns during NSTs, reporting non-reactive results or decreased movement promptly.
- Prepare neonates with IUGR for NICU admission, monitoring for hypothermia, hypoglycemia, and respiratory distress.
- Collaborate with maternal-fetal medicine specialists to develop a delivery plan for IUGR pregnancies.
Nutritional Deficiencies And Maternal Obesity
Nutritional deficiencies and maternal obesity significantly impact maternal and fetal health, contributing to congenital anomalies, obstetric complications, and long-term health risks.
14.1 Folic Acid and Iron Deficiencies
Deficiencies in folic acid and iron are common during pregnancy and are associated with significant maternal and fetal complications if not addressed.
- Folic Acid Deficiency: Folic acid is essential for DNA synthesis and neural tube closure during early fetal development.
- Maternal Effects: Deficiency may contribute to megaloblastic anemia, increasing fatigue and risk of preeclampsia.
- Fetal/Neonatal Effects: Folic acid deficiency is strongly associated with neural tube defects (NTDs), such as spina bifida and anencephaly, particularly when deficient in the first 28 days post-conception.
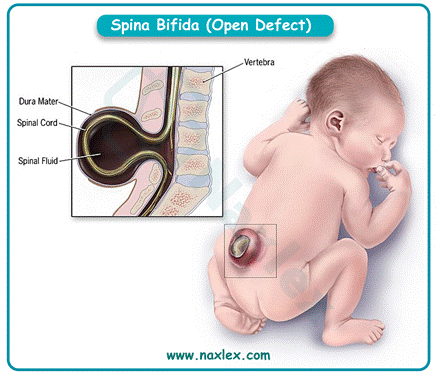
- Management: Supplementation with 400–800 mcg of folic acid daily is recommended preconceptionally and throughout pregnancy. High-risk patients (e.g., history of NTDs) may require 4 mg daily.
- Iron Deficiency: Iron is critical for hemoglobin synthesis and oxygen transport, with increased demands during pregnancy due to expanded maternal blood volume and fetal needs.
- Maternal Effects: Iron deficiency anemia (IDA) leads to fatigue, pallor, and increased risk of preterm labor and postpartum hemorrhage.
- Fetal/Neonatal Effects: IDA is associated with low birth weight, preterm birth, and impaired neonatal iron stores, increasing the risk of developmental delays.
- Management: Iron supplementation (30–60 mg daily) is recommended, with dietary counseling to include iron-rich foods (e.g., lean meats, spinach).
Nursing Insights
- Educate patients on the importance of daily folic acid supplementation, starting preconceptionally, to prevent NTDs.
- Assess hemoglobin and hematocrit levels at the initial prenatal visit and at 28 weeks to detect IDA, initiating supplementation as needed.
- Counsel patients on consuming iron-rich foods and co-ingesting vitamin C to enhance iron absorption.
- Monitor for signs of anemia, such as fatigue, pallor, or tachycardia, and educate patients to report these symptoms.
- Advise patients to take iron supplements on an empty stomach to improve absorption, while monitoring for gastrointestinal side effects.
14.2 Obesity-Related Risks
Maternal obesity, defined as a pre-pregnancy body mass index (BMI) ≥30 kg/m², increases the risk of obstetric complications and fetal anomalies due to metabolic and inflammatory changes.
- Maternal Effects: Obesity is associated with an increased incidence of gestational diabetes mellitus (GDM), preeclampsia, and cesarean delivery due to macrosomia or labor dystocia.
- Obese women are at higher risk for postpartum hemorrhage and wound infections post-cesarean.
- Fetal/Neonatal Effects: Fetal macrosomia (>4,000 g) increases the risk of shoulder dystocia, birth trauma, and neonatal hypoglycemia.
- Obesity is linked to congenital anomalies, such as neural tube defects and congenital heart defects, independent of folic acid status.
- Long-term, infants of obese mothers are at increased risk for childhood obesity and metabolic syndrome.
- Management: Weight management through dietary counseling and safe exercise is recommended. Screening for GDM at 24–28 weeks and close monitoring for preeclampsia are critical.
Nursing Insights
- Calculate pre-pregnancy BMI at the initial prenatal visit to identify obese patients and tailor interventions.
- Provide nutritional counseling to promote a balanced diet and appropriate weight gain (11–20 lbs for obese women, per IOM guidelines).
- Screen for GDM using the one-hour glucose tolerance test at 24–28 weeks, with earlier screening for high-risk obese patients.
- Monitor blood pressure and proteinuria at each visit to detect early signs of preeclampsia in obese patients.
- Prepare for potential shoulder dystocia during delivery, ensuring availability of additional staff and equipment.
Summary
- The management of risk factors affecting pregnancy outcomes is a cornerstone of maternal-newborn nursing, requiring comprehensive assessment, vigilant monitoring, and tailored interventions to optimize maternal and fetal health.
- Key risk factors include maternal age, preexisting medical conditions, infectious diseases, substance use, environmental exposures, psychosocial and socioeconomic factors, obstetric and fetal complications, and nutritional deficiencies or maternal obesity.
- Adolescent pregnancies (ages 10–19) are associated with increased risks of preterm birth, low birth weight, and cephalopelvic disproportion due to physiological immaturity and psychosocial challenges.
- Advanced maternal age (≥35 years) elevates the risk of chromosomal abnormalities, gestational diabetes mellitus (GDM), and preeclampsia due to declining oocyte quality and maternal comorbidities.
- Preexisting conditions such as diabetes mellitus, hypertension, and thyroid disorders necessitate meticulous management to prevent congenital anomalies, macrosomia, intrauterine growth restriction (IUGR), and preterm birth.
- TORCH infections (toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, and others like syphilis) and sexually transmitted infections (STIs) like chlamydia, gonorrhea, and HIV can cause congenital anomalies and neonatal sepsis, requiring routine screening and timely treatment.
- Group B Streptococcus (GBS) colonization, present in 10–30% of pregnant women, demands intrapartum antibiotic prophylaxis to prevent neonatal sepsis. Substance use, including tobacco, alcohol, and illicit drugs, is highly teratogenic, leading to fetal alcohol spectrum disorders, neonatal abstinence syndrome, and IUGR, while environmental toxins like lead and mercury increase risks of miscarriage and developmental delays.
- Psychosocial factors, such as intimate partner violence (IPV) and depression, and socioeconomic barriers, including food insecurity, contribute to poor prenatal care adherence and adverse outcomes.
- Obstetric complications like multiple gestation, placenta previa, placental abruption, and IUGR require frequent fetal surveillance and preparedness for cesarean delivery. Nutritional deficiencies in folic acid and iron are linked to neural tube defects and anemia, respectively, while maternal obesity increases risks of GDM, preeclampsia, and shoulder dystocia.
- Nurses play a critical role in screening, educating, and coordinating multidisciplinary care to address these risks, using tools like the Edinburgh Postnatal Depression Scale, non-stress tests (NSTs), and ultrasound to ensure early detection and intervention.
- By addressing these factors holistically, nurses can significantly reduce maternal and neonatal morbidity and mortality, promoting optimal pregnancy outcomes.
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Maternal Risk Factors
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


