Please set your exam date
Fertilization and Implantation, Fetal Environment
Study Questions
Practice Exercise
Which of the following best describes the function of the trophoblast?
Explanation
Trophoblast is a specialized cell layer forming the outer layer of the blastocyst. Its main role is to support implantation, establish placental circulation, and produce essential hormones like hCG. These cells actively invade the endometrium to anchor the embryo and form part of the chorionic villi, critical for maternal-fetal nutrient exchange.
Rationale for correct answers
C. The trophoblast invades maternal endometrial tissue during implantation to establish uteroplacental circulation. This invasion allows the embryo to embed securely into the uterus and initiate formation of the placenta through chorionic villi development. This invasion is essential for successful pregnancy continuation.
Rationale for incorrect answers
A. The embryonic heart and neural tube are derived from the mesoderm and ectoderm of the embryonic disc, not the trophoblast. These structures originate from the inner cell mass during gastrulation, not the trophoblastic layer.
B. Amniotic fluid is initially produced by the amniotic membrane and later by fetal urine. The trophoblast contributes to chorionic membrane formation but not directly to early amniotic fluid production.
D. Limb formation is controlled by the embryonic mesoderm, specifically the lateral plate mesoderm and apical ectodermal ridge. The trophoblast plays no role in morphogenesis of limbs.
Take home points
- Trophoblast cells are responsible for endometrial invasion during implantation.
- They form the early chorionic villi necessary for placenta formation.
- The trophoblast is hormonally active, secreting hCG to maintain corpus luteum function.
- It does not contribute to embryonic organ or limb development.
In reviewing the mechanism of trophoblast invasion. Which of the following enzymes is released by syncytiotrophoblasts?
Explanation
Syncytiotrophoblasts are the outer multinucleated layer of trophoblast cells involved in implantation, maternal tissue invasion, and placental development. These cells secrete enzymes that degrade the extracellular matrix (ECM), allowing the embryo to embed into the endometrial stroma. Key enzymes include matrix metalloproteinases (MMPs) and hyaluronidase, which specifically target structural proteins and glycosaminoglycans of the ECM.
Rationale for correct answers
C. Matrix metalloproteinases and hyaluronidase are secreted by syncytiotrophoblasts to degrade collagen, fibronectin, laminin, and hyaluronic acid in the maternal endometrium. This degradation enables proper trophoblastic invasion, anchoring of the blastocyst, and remodeling of maternal spiral arteries.
Rationale for incorrect answers
A. Collagenase is a type of MMP but aldolase is a glycolytic enzyme, not involved in tissue invasion. Syncytiotrophoblasts do not secrete aldolase as part of the implantation process.
B. Acrosin and amylase are secreted by the sperm. Acrosin facilitates penetration of the zona pellucida during fertilization. Amylase digests carbohydrates and is not involved in implantation or ECM degradation.
D. Protease inhibitors oppose proteolytic activity and would hinder trophoblast invasion. Histidine kinase is part of bacterial two-component regulatory systems and has no role in mammalian implantation.
Take home points
- Syncytiotrophoblasts secrete MMPs and hyaluronidase for ECM breakdown.
- Trophoblast invasion is crucial for proper placental development.
- Acrosin is sperm-derived, not secreted by trophoblasts.
- Inhibitors of proteolysis would impair implantation.
A nurse is educating a group of students on decidualization. Which of the following regions gives rise to the maternal part of the placenta?
Explanation
Decidualization is the transformation of endometrial stromal cells into secretory decidual cells under the influence of progesterone after ovulation. It prepares the uterus for implantation, supports trophoblast invasion, and contributes to placental development. The decidua basalis, located directly beneath the implanted blastocyst, gives rise to the maternal portion of the placenta. It interacts with invading trophoblasts and undergoes significant vascular and cellular remodeling.
Rationale for correct answers
B. Decidua basalis lies directly beneath the implanted embryo and is the site of trophoblast invasion. It contains maternal blood vessels and stromal cells that become part of the placental intervillous space. It forms the maternal contribution to the placenta.
Rationale for incorrect answers
A. Decidua capsularis is the thin layer of endometrium that overlies the implanted embryo. It does not contribute to placental structure but instead stretches as the gestational sac expands, eventually fusing with the parietalis.
C. Decidua parietalis lines the remaining endometrial surface not directly involved in implantation. It plays no structural role in placental formation and becomes compressed as the gestational sac enlarges.
D. The endometrial myometrium is the muscular layer beneath the endometrium. It does not undergo decidualization and has no direct role in placental formation. It provides contractile support during labor but is not part of the placenta.
Take home points
- Decidua basalis forms the maternal part of the placenta.
- Decidua capsularis surrounds the embryo but doesn’t contribute to placenta.
- Decidua parietalis lines non-implantation sites of the uterus.
- Myometrium is muscular and does not participate in decidualization.
A nurse is teaching about factors that hinder sperm transport. Which of the following would likely impair sperm progression?
Select all that apply
Explanation
Sperm transport is the coordinated movement of sperm from the vagina to the ampulla of the fallopian tube. It is influenced by vaginal pH, cervical mucus, uterine contractions, and sperm motility. Sperm require alkaline pH (7.2–8.0), thin estrogen-dominated cervical mucus, and forward progressive motility (>32% per WHO) to reach the site of fertilization efficiently.
Rationale for correct answers
A. Acidic vaginal pH (below 7.0) reduces sperm viability. Normal vaginal pH is approximately 3.8–4.5, but semen buffers it temporarily. Persistently acidic conditions damage sperm membranes and impair motility, hindering progression through the cervix.
D. Blockage of cervical crypts prevents storage and guidance of sperm through the cervical canal. These crypts secrete mucus and facilitate sperm transport toward the uterus. Obstruction disrupts this path and impairs sperm ascent.
E. Decreased sperm motility, defined as <40% total motility or <32% progressive motility per WHO criteria, prevents effective movement through cervical mucus and uterine cavity, reducing chances of fertilization.
Rationale for incorrect answers
B. Thick cervical mucus does not occur during ovulation. Under the influence of estrogen, cervical mucus becomes thin, watery, and stretchable (spinnbarkeit), which facilitates sperm penetration. Thick mucus is seen in the luteal phase due to progesterone dominance and is not a feature of ovulation.
C. Uterine contractions post-intercourse aid sperm transport by creating suction-like currents that move sperm toward the uterine tubes. Oxytocin and prostaglandins in semen stimulate these contractions, enhancing fertility rather than hindering it.
Take home points
- Acidic vaginal pH reduces sperm survival and transport.
- Cervical crypt blockage impairs sperm guidance into the uterus.
- Poor sperm motility limits fertilization potential.
- Ovulatory cervical mucus and uterine contractions support sperm migration.
Which of the following are correct pairings of decidua regions with their descriptions?
Select all that apply
Explanation
Decidua is the transformed endometrium of pregnancy, influenced by progesterone, which induces stromal cell differentiation, glandular hypertrophy, and vascular remodeling. It is divided into distinct regions based on location relative to the embryo. Decidua basalis, capsularis, and parietalis each have structural and functional relevance. The thickness ranges from 5–10 mm in early gestation and supports implantation and placental development.
Rationale for correct answers
A. Decidua basalis is the region directly beneath the implanted blastocyst and forms the maternal part of the placenta. It interacts with trophoblasts and contributes to the intervillous space and placental bed.
B. Decidua capsularis is the part of the endometrium that overlies and encloses the implanted embryo. It stretches as the embryo grows and later fuses with the decidua parietalis.
C. Decidua parietalis lines the remaining uterine cavity not occupied by the embryo. By the end of the first trimester, it fuses with the expanding decidua capsularis as the chorionic sac fills the uterine space.
Rationale for incorrect answers
D. Decidua spongiosa is not a recognized anatomical subdivision of the decidua. The term "spongiosa" typically refers to histologic zones in the endometrium or myometrium but not a decidual region associated with implantation or placental function.
E. Decidua amnion is not a valid anatomical term. The amnion is a fetal membrane derived from epiblast cells and does not originate from decidual tissue. Chorionic villi are surrounded by the chorion and bathed in maternal blood, not lined by a so-called "decidua amnion".
Take home points
- Decidua basalis is the maternal placenta-forming region.
- Decidua capsularis surrounds the implanted embryo.
- Decidua parietalis lines the rest of the uterine cavity.
- Terms like decidua spongiosa and decidua amnion are incorrect in placental anatomy.
Practice Exercise 2
Which of the following statements indicates understanding of the amnion?
Explanation
Amnion is a fetal membrane derived from the epiblast that forms the amniotic cavity, which surrounds and cushions the embryo. It is lined by amnioblasts, separates from the cytotrophoblast, and expands with accumulating amniotic fluid, which reaches 500–1000 mL at term. Normal fluid index is 5–25 cm. It protects against trauma, infection, and desiccation.
Rationale for correct answers
C. The amnion originates from the epiblast during the second week of development. A cavity forms within the epiblast, bordered by amnioblasts, leading to the formation of the amniotic cavity. This structure eventually encloses the entire embryo and contributes to fluid regulation.
Rationale for incorrect answers
A. The amnion is not derived from the syncytiotrophoblast. Syncytiotrophoblasts are involved in maternal tissue invasion and hCG secretion but not in amniotic membrane formation. The amnion originates from the inner cell mass specifically from the epiblast.
B. The amnion does not produce hormones like human chorionic gonadotropin. hCG is secreted by the syncytiotrophoblast starting around day 8 post-fertilization and peaks at approximately 100,000 IU/L by week 10. The amnion primarily serves a mechanical and protective function.
D. The amnion and chorion eventually fuse by week 14, obliterating the chorionic cavity. Persistence of their separation would indicate abnormal development. This fusion is necessary to complete formation of the amniotic sac surrounding the fetus.
Take home points
- The amnion originates from the epiblast and forms the amniotic cavity.
- It plays a structural and fluid-regulatory role, not hormonal.
- Syncytiotrophoblasts are unrelated to amnion formation.
- Amnion and chorion fuse during the second trimester.
Which of the following fetal conditions is a possible cause of polyhydramnios?
Explanation
Polyhydramnios is the pathological accumulation of amniotic fluid exceeding 2000 mL or an amniotic fluid index (AFI) >24 cm. It results from impaired fetal swallowing, increased fetal urination, or maternal-fetal interface dysfunction. Normal AFI ranges from 5–25 cm. Fetal neurological or gastrointestinal abnormalities commonly disrupt fluid regulation, leading to excessive accumulation.
Rationale for correct answers
B. Anencephaly causes impaired fetal swallowing due to the absence of major portions of the brain and skull. This results in an inability to recycle amniotic fluid through the gastrointestinal system, leading to progressive accumulation. Anencephaly is a common cause of severe polyhydramnios, especially in the second trimester.
Rationale for incorrect answers
A. Posterior urethral valves cause bladder outlet obstruction, leading to decreased fetal urination. This reduces amniotic fluid production, often causing oligohydramnios, not polyhydramnios. Ultrasound findings typically show a distended bladder with reduced fluid.
C. Renal agenesis results in absent fetal kidneys and thus complete lack of urine production, which is the major source of amniotic fluid in the second half of pregnancy. This condition leads to severe oligohydramnios, not polyhydramnios, and is associated with Potter sequence.
D. Intrauterine growth restriction (IUGR) is often associated with placental insufficiency and reduced fetal urine output, leading to oligohydramnios. It is rarely associated with increased amniotic fluid and more commonly presents with AFI <5 cm.
Take home points
- Anencephaly leads to polyhydramnios due to impaired fetal swallowing.
- Posterior urethral valves and renal agenesis cause oligohydramnios.
- Polyhydramnios is defined as AFI >24 cm or fluid volume >2000 mL.
- Fetal GI and CNS anomalies are major causes of polyhydramnios.
Which of the following best describes a function of the chorionic villi?
Explanation
Chorionic villi are vascular projections of the chorion formed by proliferating cytotrophoblasts and invading syncytiotrophoblasts. They extend into the decidua basalis, creating the placental interface for nutrient and gas exchange. These structures contain fetal capillaries surrounded by trophoblastic tissue and allow transfer of oxygen, carbon dioxide, glucose, amino acids, and electrolytes. Normal fetal oxygen saturation ranges between 30–70% in the umbilical vein.
Rationale for correct answers
D. Chorionic villi contain fetal capillaries surrounded by trophoblasts, facilitating the bidirectional transfer of gases and nutrients between maternal blood in the intervillous space and fetal circulation. This exchange supports fetal growth and development and begins by the end of the third week of gestation.
Rationale for incorrect answers
A. Luteinizing hormone is not secreted by chorionic villi. Instead, human chorionic gonadotropin (hCG) is produced by syncytiotrophoblasts to maintain the corpus luteum. LH is a pituitary hormone and has no role in early placental function.
B. The fetal heartbeat is initiated by the sinoatrial node in the primitive heart tube, not by chorionic villi. Cardiac activity begins at approximately 5.5–6 weeks of gestation and is driven by intrinsic pacemaker activity within the developing myocardium.
C. The yolk sac is an endoderm-derived structure, and its outer layer is formed by extraembryonic mesoderm and endoderm, not chorionic villi. Chorionic villi are entirely separate from the yolk sac and serve different physiological roles.
Take home points
- Chorionic villi are the site of maternal-fetal exchange.
- They develop from cytotrophoblast and syncytiotrophoblast layers.
- They are not involved in cardiac or hormonal functions.
- They are distinct from the yolk sac and its components.
A nurse is reviewing normal contents of amniotic fluid. Which of the following are expected components? Select all that apply
Explanation
Amniotic fluid is a dynamic, sterile, and slightly alkaline liquid that surrounds the fetus, produced by fetal urine, pulmonary secretions, and amnion. It contains electrolytes, proteins, lipids, and desquamated fetal cells. Normal volume is 500–1000 mL at term, with an amniotic fluid index (AFI) of 5–25 cm. Its functions include protection, fluid balance, and developmental support.
Rationale for correct answers
A. Vernix caseosa, a white, cheesy substance produced by fetal sebaceous glands, is found in amniotic fluid starting from the third trimester. It consists of lipids, proteins, and desquamated skin, and floats in the amniotic fluid as the fetus sheds it.
C. Fetal skin cells, also called squamous cells, are routinely present in amniotic fluid, especially as gestation progresses. These desquamated cells are important for diagnostic procedures such as amniocentesis and fetal karyotyping.
D. Urea is a nitrogenous waste product derived from fetal metabolism and is excreted in fetal urine, which significantly contributes to amniotic fluid composition after 16 weeks. Normal concentrations rise with gestational age and help indicate fetal renal function.
Rationale for incorrect answers
B. Hemoglobin A1C is a glycated hemoglobin used to assess maternal blood glucose control. It is not a component of amniotic fluid. Maternal blood is separate from the amniotic compartment, and A1C reflects long-term maternal glycemia, not fetal or fluid content.
E. Amylase, a digestive enzyme, is not normally found in significant amounts in amniotic fluid. It is produced by the fetal pancreas and salivary glands later in gestation but does not accumulate in measurable concentrations in the fluid under normal physiological conditions.
Take home points
- Vernix caseosa and fetal skin cells are normal amniotic components.
- Urea in fluid reflects fetal renal output and maturity.
- Hemoglobin A1C is a maternal blood marker, not found in fluid.
- Enzymes like amylase are not typically present in normal amniotic fluid.
A nurse is assessing a client with confirmed polyhydramnios. Which of the following risks should the nurse anticipate? Select all that apply
Explanation
Polyhydramnios is excessive amniotic fluid accumulation with amniotic fluid index (AFI) >24 cm or single deepest pocket >8 cm. It can result from fetal anomalies, maternal diabetes, or idiopathic causes. It increases uterine distention, raising the risk of preterm labor, malpresentations, and maternal dyspnea. Normal AFI ranges between 5–25 cm.
Rationale for correct answers
A. Uterine overdistention from excessive amniotic fluid stretches the myometrium, which increases irritability and sensitivity to oxytocin, promoting early and uncoordinated uterine contractions. This significantly raises the risk of preterm labor, particularly when AFI exceeds 30 cm.
B. Excess fluid increases intrauterine pressure and may allow umbilical cord descent through the cervical os when membranes rupture, especially in cases of high fetal station or malpresentation. This sudden gush of fluid may carry the cord ahead of the presenting part.
E. Polyhydramnios causes rapid uterine enlargement, elevating the diaphragm and compressing maternal lungs. This can result in restrictive pulmonary mechanics and maternal dyspnea, particularly in the third trimester. Pulmonary function tests often show reduced forced vital capacity.
Rationale for incorrect answers
C. Shoulder dystocia is commonly associated with macrosomia, gestational diabetes, or post-term pregnancy, not polyhydramnios. Polyhydramnios more commonly leads to malpresentations such as breech or transverse lie due to excessive fetal mobility, rather than shoulder entrapment.
D. Post-term delivery is not associated with polyhydramnios. In fact, polyhydramnios often precipitates earlier delivery due to preterm labor, premature rupture of membranes, or the need for induction because of maternal or fetal compromise.
Take home points
- Polyhydramnios increases risk for preterm labor and cord prolapse.
- Uterine overdistention causes maternal dyspnea from diaphragmatic elevation.
- Shoulder dystocia is not related to amniotic fluid volume.
- Post-term pregnancy is less likely due to premature delivery from complications.
Practice Exercise 3
The passive immunity a newborn receives from the mother is primarily due to the placental transfer of which of the following immunoglobulins?
Explanation
Passive immunity in the newborn is conferred primarily by the transplacental transfer of maternal antibodies during the third trimester, providing immediate but temporary protection. The predominant immunoglobulin transferred is immunoglobulin G (IgG), which crosses the placenta via Fc receptors. Normal maternal IgG serum concentration ranges from 700 to 1600 mg/dL. IgG levels in the neonate can be 50–70% of maternal levels at birth, offering protection until the infant’s own immune system matures.
Rationale for correct answers
C. Immunoglobulin G (IgG) is the only immunoglobulin class that efficiently crosses the placenta by active transport via the neonatal Fc receptor. This transfer mainly occurs after 28 weeks of gestation, increasing significantly in the last trimester, conferring passive immunity against pathogens.
Rationale for incorrect answers
A. Immunoglobulin A (IgA) is the major antibody found in colostrum and breast milk, providing mucosal immunity postnatally but does not cross the placenta in significant amounts.
B. Immunoglobulin M (IgM) is a large pentameric molecule that does not cross the placental barrier due to its size; neonatal IgM synthesis begins after birth, indicating in utero infection if detected.
D. Immunoglobulin D (IgD) is present in very low concentrations in serum and is primarily involved in B-cell receptor function. It does not cross the placenta and plays no role in passive immunity.
Take home points
- IgG is the main immunoglobulin transferred transplacentally.
- Passive immunity via IgG protects the newborn until active immunity develops.
- IgA provides mucosal protection through breastfeeding, not placental transfer.
- Presence of IgM in newborn suggests in utero infection, not passive transfer.
A nurse examines the umbilical cord and notes the presence of two umbilical arteries and one umbilical vein. The nurse correctly documents this finding as which of the following?
Explanation
The umbilical cord typically consists of two umbilical arteries and one umbilical vein, embedded in Wharton’s jelly. This structure facilitates fetal-maternal exchange, carrying oxygenated blood via the vein and deoxygenated blood via the arteries. Normal umbilical cord length ranges from 40 to 70 cm at term, and a normal cord has a diameter of 1 to 2 cm. Variations can indicate fetal anomalies or complications but the presence of two arteries and one vein is the standard healthy anatomy.
Rationale for correct answers
C. The presence of two umbilical arteries and one vein is the normal anatomical structure of the umbilical cord. This arrangement is essential for adequate fetal circulation. The two arteries carry deoxygenated blood from the fetus to the placenta, while the single vein returns oxygenated blood to the fetus. This finding indicates normal umbilical cord development.
Rationale for incorrect answers
A. An abnormal finding requiring immediate notification is typically a single umbilical artery (SUA), which occurs in about 1% of pregnancies. The presence of two arteries is normal and does not require urgent intervention.
B. A single umbilical artery is associated with increased risk of congenital anomalies such as cardiac, renal, or chromosomal abnormalities. However, two arteries do not signify this increased risk.
D. Velamentous cord insertion refers to the umbilical vessels inserting into the fetal membranes rather than the placental mass and is often associated with single umbilical artery or other anomalies but not the typical two-artery one-vein configuration.
Take home points
- Two umbilical arteries and one vein represent normal umbilical cord anatomy.
- Single umbilical artery is the abnormal variant linked to fetal anomalies.
- Normal umbilical cord is 40–70 cm long and 1–2 cm in diameter at term.
- Velamentous cord insertion is a separate abnormality unrelated to artery number.
The fetal surface of the placenta is called the "Shiny Schultze" is covered by which of the following membranes?
Explanation
The fetal surface of the placenta, also called the "shiny Schultze", is the smooth, glistening side facing the fetus. It is covered by the amnion, a thin, transparent membrane derived from the epiblast. This membrane encloses the amniotic cavity and protects the fetus and placenta. The chorion and decidua basalis are other placental layers but do not cover the fetal surface directly. Normal placental weight at term is approximately 500 grams, and the fetal surface area averages 15–20 cm in diameter.
Rationale for correct answers
B. The amnion covers the fetal surface of the placenta, forming a smooth, shiny membrane known as the Schultze presentation. It originates from the epiblast and provides a protective barrier for the fetus and placenta against mechanical injury and infection. This membrane is avascular and adheres tightly to the chorion but is distinct from it.
Rationale for incorrect answers
A. The chorion is the outer fetal membrane that, together with the amnion, forms the fetal sac but it primarily covers the outer surface facing the maternal side or lies beneath the decidua. It does not form the shiny fetal surface of the placenta.
C. The decidua basalis is the maternal component of the placenta, forming the maternal-fetal interface on the uterine side, not the fetal surface.
D. The trophoblast forms the outer layer of the blastocyst and contributes to placental formation but does not cover the fetal surface as a membrane; it is part of the placental villous structure.
Take home points
- The amnion covers the fetal surface of the placenta, creating the shiny Schultze appearance.
- The chorion surrounds the amnion and contributes to the fetal membranes but does not cover the fetal surface directly.
- The decidua basalis is the maternal placental layer, not involved in fetal surface covering.
- The trophoblast forms placental villi but is not a surface membrane.
Which of the following are functions of the placenta? Select all that apply.
Explanation
The placenta is an essential fetal organ responsible for nutrient metabolism, storage, and transfer, as well as waste elimination. It synthesizes important macronutrients like glycogen and fatty acids to support fetal energy and growth. It also stores fat-soluble vitamins (A, D, E, K) critical for fetal development. The placenta maintains a selective barrier, facilitating exchange without retaining fetal wastes.
Rationale for correct answers
A. The placenta synthesizes glycogen, which acts as an energy reserve for the fetus, especially important during periods of increased metabolic demand. Glycogen synthesis in the placenta supports fetal glucose supply.
B. Fatty acids synthesized by the placenta are vital for fetal cellular growth, particularly in neural and organ development. These fatty acids supplement maternal supply and are essential for membrane formation.
D. Fat-soluble vitamins (A, D, E, K) are stored in the placenta and regulated for fetal use. These vitamins are crucial for antioxidative protection, bone development, and coagulation in the fetus.
Rationale for incorrect answers
C. The placenta does not catabolize maternal proteins to provide amino acids; instead, amino acids are transported intact from maternal circulation to the fetus. Protein catabolism occurs mainly in maternal tissues.
E. The placenta does not retain fetal waste products; it facilitates the removal of fetal wastes such as urea and carbon dioxide by transferring them to maternal circulation for excretion. Retention would be harmful to fetal health.
Take home points
- Placenta synthesizes glycogen and fatty acids to support fetal energy and growth.
- It stores fat-soluble vitamins necessary for fetal development.
- Amino acids cross the placenta via transport, not from placental protein catabolism.
- The placenta facilitates fetal waste excretion; it does not retain wastes.
A nurse reviews placental variants. Which of the following are expected eventualities? Select all that apply
Explanation
Placental variants involve anatomical deviations in placental structure and attachment affecting fetal-maternal exchange. Important variants include succenturiate lobes and battledore placenta, which alter placental morphology and cord insertion. These variants impact delivery outcomes due to risks like retained tissue, vascular complications, and possible fetal effects. A normal placenta typically has a smooth, uniform margin measuring 15–20 cm in diameter.
Rationale for correct answers
A. A succenturiate lobe is an accessory placental lobe connected to the main placenta by blood vessels. This lobe may remain in the uterus post-delivery, increasing risk for retained placental tissue and postpartum hemorrhage.
B. Battledore placenta refers to a marginal umbilical cord insertion near the placental edge. This variant can affect blood flow distribution but is often benign. It is named for the paddle-like shape resembling a battledore.
D. The succenturiate lobe is anatomically connected to the main placenta via vascular structures, which may be prone to rupture or thrombosis, potentially compromising fetal circulation or causing hemorrhage.
Rationale for incorrect answers
C. The margin of a placenta with variants like succenturiate lobe or battledore insertion is often irregular, not smooth or uniform. A smooth, uniform margin describes a normal placenta, not variants.
E. Battledore placenta does not always cause fetal growth restriction; many cases are asymptomatic without impacting fetal growth. Growth restriction is more related to other placental insufficiencies.
Take home points
- Succenturiate lobes increase risk of retained placental tissue postpartum.
- Battledore placenta involves marginal cord insertion, affecting placental blood flow.
- The succenturiate lobe is connected to the main placenta by blood vessels, posing hemorrhage risk.
- Normal placental margins are smooth and uniform; variants disrupt this morphology.
Comprehensive Questions
Which of the following statements accurately describes the role of the amnion at 12 weeks gestation?
Explanation
The amnion is a thin, transparent membrane that forms the innermost layer of the fetal membranes, creating a protective sac filled with amniotic fluid. By 12 weeks gestation, the amnion has expanded to enclose the fetus completely, providing a cushioning environment, temperature regulation, and protection against infection and mechanical injury. Amniotic fluid volume normally ranges from 50 ml at 12 weeks to approximately 800 ml at term. The amnion does not produce hormones or directly facilitate nutrient exchange, which is the role of the placenta.
Rationale for correct answers
B. The amnion forms a protective sac that contains amniotic fluid, which cushions the fetus and permits movement. At 12 weeks, this sac is fully formed, surrounding the embryo/fetus with fluid essential for normal development and mechanical protection.
Rationale for incorrect answers
A. The amnion does not produce hormones; hormone production to maintain pregnancy is primarily by the trophoblast and placenta, including human chorionic gonadotropin and progesterone.
C. Nutrient exchange occurs at the placenta via chorionic villi, not the amnion, which acts as a barrier and container for amniotic fluid but does not participate in exchange functions.
D. The umbilical cord develops from the connecting stalk and allantois, not from the amnion. The amnion lines the amniotic cavity but does not differentiate into the umbilical cord.
Take home points
- The amnion forms a protective sac filled with amniotic fluid by 12 weeks gestation.
- Amniotic fluid cushions and protects the fetus, volume increases from 50 ml at 12 weeks to 800 ml at term.
- Hormone production for pregnancy maintenance occurs in the placenta, not the amnion.
- Nutrient and gas exchange occurs through the placenta, not the amnion.
Which of the following is the key event that characterizes the acrosomal reaction?
Explanation
The acrosomal reaction is a crucial event in human fertilization involving the release of hydrolytic enzymes from the sperm’s acrosome. These enzymes allow the sperm to penetrate the zona pellucida, a glycoprotein-rich extracellular matrix surrounding the oocyte. Hyaluronidase and acrosin are the two main enzymes involved. The reaction begins upon binding of the sperm to the ZP3 glycoprotein receptor on the zona pellucida. The acrosomal reaction does not involve genetic fusion but is essential to reach the oocyte membrane. This reaction precedes cortical reaction and oocyte activation.
Rationale for correct answers
B. The acrosomal reaction is defined by the release of hydrolytic enzymes like acrosin and hyaluronidase from the sperm’s acrosomal cap. These enzymes digest the cumulus oophorus and the zona pellucida, allowing sperm to reach the oocyte membrane. This event is molecularly triggered by the binding of sperm to ZP3 receptors on the zona pellucida.
Rationale for incorrect answers
A. Fusion of the male and female pronuclei marks syngamy, which is the final step of fertilization after the sperm has already entered the oocyte. This event occurs after both the acrosomal and cortical reactions and does not define the acrosomal reaction itself.
C. A rapid depolarization of the oocyte membrane is part of the “fast block to polyspermy,” which is associated with the cortical reaction, not the acrosomal reaction. This block prevents multiple sperm from fertilizing the oocyte after one sperm has succeeded, but it happens after the acrosomal reaction is complete.
D. Migration of sperm through the cervical mucus and uterus is a part of sperm transport and capacitation, which occur prior to the acrosomal reaction. This is necessary for the sperm to even reach the site of fertilization, but it is not the defining event of the acrosomal reaction.
Take home points
- The acrosomal reaction is triggered by sperm binding to ZP3 in the zona pellucida.
- Hydrolytic enzymes like acrosin are released to penetrate the zona pellucida.
- The acrosomal reaction is essential for fertilization but occurs before sperm-oocyte membrane fusion.
- It is distinct from the cortical reaction and sperm transport.
Which of the following structures is formed from the proliferation of chorionic villi adjacent to the decidua basalis?
Explanation
The chorion frondosum is the part of the chorion characterized by proliferation of vascularized villi adjacent to the decidua basalis, which contributes to the formation of the fetal placenta. These villi undergo extensive branching and vascularization to establish maternal-fetal exchange. In contrast, the chorion laeve is the smooth, avascular part away from the decidua basalis. Proper development of the chorion frondosum is essential for adequate placental function, supporting fetal nutrition and gas exchange throughout pregnancy.
Rationale for correct answers
B. The chorion frondosum forms from the proliferation of chorionic villi adjacent to the decidua basalis, developing into the functional fetal placenta with a dense vascular network enabling exchange of nutrients, gases, and waste. This structure is essential for sustaining fetal growth.
Rationale for incorrect answers
A. The chorion laeve, or smooth chorion, forms from villi on the side of the chorion away from the decidua basalis; these villi regress and become avascular, so it does not contribute to placental formation.
C. The yolk sac is an early embryonic structure involved in initial hematopoiesis and nutrient transfer but is not formed from chorionic villi and is distinct from the chorion.
D. The umbilical cord develops from the connecting stalk and contains blood vessels connecting fetus to placenta but is not formed by chorionic villi proliferation adjacent to the decidua basalis.
Take home points
- The chorion frondosum is the vascularized region of chorionic villi adjacent to the decidua basalis forming the fetal placenta.
- The chorion laeve is avascular and forms the smooth chorion on the opposite side of the placenta.
- Proper placental development depends on chorionic villi proliferation and vascularization in the chorion frondosum.
- The yolk sac and umbilical cord are distinct structures not formed from chorionic villi adjacent to decidua basalis.
The majority of the amniotic fluid in the third trimester is contributed by which of the following?
Explanation
Amniotic fluid volume is tightly regulated and serves multiple functions, including cushioning the fetus, facilitating movement, and allowing lung development. In the third trimester, the majority of amniotic fluid is produced by fetal urine excreted into the amniotic cavity and lung fluid secreted by the fetal respiratory tract. Normal amniotic fluid volume ranges between 500 to 1000 mL at term. Fluid turnover involves swallowing and absorption by the fetus as well as contributions from membranes and maternal sources.
Rationale for correct answers
C. Fetal urine production significantly increases in the third trimester, contributing the bulk of amniotic fluid volume. Additionally, fetal lungs secrete fluid that mixes with the amniotic fluid, ensuring appropriate volume and composition. This combined secretion accounts for the majority of amniotic fluid after 28 weeks gestation.
Rationale for incorrect answers
A. Diffusion of fluid from maternal blood across the placental membranes contributes minimally to amniotic fluid volume, especially in the third trimester, as fetal urine becomes predominant.
B. Secretions from the fetal gastrointestinal tract do not contribute significantly to amniotic fluid volume; rather, swallowed amniotic fluid is absorbed, not secreted into the cavity.
D. Transudation from fetal skin is significant only early in gestation before keratinization, which is complete by about 20 weeks, greatly reducing this source by the third trimester.
Take home points
- The majority of third trimester amniotic fluid is produced by fetal urine and lung fluid secretions.
- Amniotic fluid volume ranges from 500 to 1000 mL near term and is critical for fetal development.
- Maternal diffusion and fetal skin transudation contribute minimally after mid-gestation.
- Fetal swallowing and absorption regulate amniotic fluid volume alongside production.
After a single sperm successfully enters the oocyte cytoplasm, the completion of fertilization results in which of the following?
Explanation
Fertilization is the process whereby a single sperm penetrates the oocyte cytoplasm, initiating the fusion of genetic material. This event results in the formation of a diploid zygote through the fusion of the male and female pronuclei. Fertilization triggers the completion of the oocyte's second meiotic division and initiates the first mitotic divisions. The zygote contains 46 chromosomes (23 pairs), restoring the diploid number essential for normal embryogenesis.
Rationale for correct answers
C. After sperm entry, the male and female pronuclei migrate towards each other and fuse, creating a single diploid nucleus within the zygote. This fusion marks the true completion of fertilization and the genetic unification necessary for embryonic development.
Rationale for incorrect answers
A. The formation of a blastocyst, containing an inner cell mass and trophoblast, occurs several days post-fertilization during the blastocyst stage, not immediately upon sperm-oocyte fusion.
B. Differentiation of the morula into an embryo occurs after several rounds of cleavage and compaction, occurring days after fertilization, thus not at the immediate completion of fertilization.
D. Rapid mitotic divisions producing blastomeres begin after fertilization but represent cleavage stages rather than the completion event of fertilization itself, which is specifically defined by pronuclear fusion.
Take home points
- Fertilization completion is defined by the fusion of male and female pronuclei forming a diploid zygote.
- The zygote contains 46 chromosomes, restoring diploidy essential for normal development.
- Subsequent events such as cleavage and blastocyst formation occur days after fertilization.
- Early embryogenesis depends on successful pronuclear fusion to initiate mitotic divisions.
Which of the following should the nurse identify as the correct number of vessels in the umbilical cord?
Explanation
The umbilical cord typically contains three vessels: two umbilical arteries and one umbilical vein. The arteries carry deoxygenated blood and waste products from the fetus to the placenta, while the single vein returns oxygenated, nutrient-rich blood from the placenta to the fetus. The normal number of vessels ensures adequate fetal circulation and nutrient exchange. Variations such as a single umbilical artery occur in about 1% of pregnancies and can be associated with congenital anomalies but are not the norm.
Rationale for correct answers
A. The presence of two arteries and one vein is the normal anatomy of the umbilical cord. The two arteries arise from the fetal internal iliac arteries and transport deoxygenated blood to the placenta, while the single vein carries oxygenated blood back to the fetus.
Rationale for incorrect answers
B. One artery and one vein represent an abnormal finding known as a single umbilical artery, which may be associated with congenital anomalies or fetal growth restriction and is not the normal anatomy.
C. Two veins and one artery is not a typical umbilical cord configuration; the umbilical cord normally has only one vein.
D. Three arteries and no veins is anatomically incorrect and incompatible with fetal survival due to lack of oxygenated blood return.
Take home points
- Normal umbilical cord anatomy includes two arteries and one vein.
- Two arteries carry deoxygenated blood from fetus to placenta; one vein returns oxygenated blood.
- Single umbilical artery is an abnormal variant with possible fetal risks.
- Proper vessel number is critical for effective fetal circulation and nutrient exchange.
Which drug property increases placental crossing?
Explanation
Placental drug transfer depends on molecular size, lipid solubility, ionization status, and protein binding of the drug. Drugs that are lipid-soluble, have low molecular weight (typically less than 500 daltons), and are non-ionized at physiological pH cross the placenta more readily via passive diffusion. Protein binding limits free drug available to cross. Most drugs cross the placenta by passive diffusion, influenced by these physicochemical properties.
Rationale for correct answers
B. Lipid-soluble and low molecular weight drugs easily diffuse through the placental lipid bilayer, facilitating passage into fetal circulation. Low molecular weight (<500 daltons) favors transfer, while lipid solubility enhances membrane permeability.
Rationale for incorrect answers
A. Large molecular weight and high protein binding reduce placental crossing. Large molecules (>1000 daltons) cannot easily cross, and high protein binding limits free drug available for transfer.
C. Ionized drugs at physiological pH are less lipid-soluble and cross the placenta poorly due to reduced membrane permeability.
D. Water-soluble and high molecular weight drugs have limited placental transfer because they cannot readily diffuse across the lipid membranes.
Take home points
- Lipid solubility and low molecular weight increase placental drug transfer.
- Protein binding and large molecular size restrict placental crossing.
- Ionized drugs cross the placenta less efficiently than non-ionized drugs.
- Passive diffusion is the main mechanism for most drugs crossing the placenta.
A fetus with prolonged oligohydramnios is at risk for which complication?
Explanation
Oligohydramnios is defined as a decreased amount of amniotic fluid, usually less than 500 mL in the third trimester or an amniotic fluid index (AFI) less than 5 cm. It results from decreased fetal urine production or increased fluid loss and is associated with fetal renal anomalies, rupture of membranes, or placental insufficiency. The amniotic fluid plays critical roles in lung development, cushioning, and movement. Prolonged oligohydramnios compromises lung growth leading to pulmonary hypoplasia, a condition where the lungs are underdeveloped, impairing neonatal respiratory function.
Rationale for correct answers
C. Pulmonary hypoplasia occurs because amniotic fluid is essential for normal lung development by maintaining fluid pressure and allowing lung expansion in utero. Prolonged oligohydramnios reduces this fluid, resulting in underdeveloped lungs with fewer alveoli and decreased pulmonary vasculature, causing respiratory insufficiency at birth.
Rationale for incorrect answers
A. Macrosomia, defined as fetal weight above 4000 grams, is unrelated to oligohydramnios and more often linked to maternal diabetes or genetic factors. Reduced amniotic fluid does not cause fetal overgrowth.
B. Shoulder dystocia is an obstetric complication caused by fetal size disproportion, often macrosomia, and is not directly caused by oligohydramnios.
D. Hyperbilirubinemia results from increased red blood cell breakdown or impaired conjugation, unrelated to amniotic fluid volume. Oligohydramnios does not predispose to bilirubin metabolism disorders.
Take home points
- Oligohydramnios is characterized by AFI less than 5 cm or fluid volume under 500 mL in the third trimester.
- Prolonged oligohydramnios leads to pulmonary hypoplasia due to impaired fetal lung development.
- Macrosomia and shoulder dystocia are unrelated to oligohydramnios but linked to fetal size.
- Hyperbilirubinemia is unrelated to amniotic fluid volume but linked to red blood cell metabolism.
Which of the following best describes the purpose of amniocentesis?
Explanation
Amniocentesis is an invasive prenatal diagnostic procedure primarily used to sample amniotic fluid for chromosomal, genetic, and fetal lung maturity assessments. Typically performed between 15 and 20 weeks’ gestation, it involves transabdominal insertion of a needle under ultrasound guidance to withdraw 15–20 mL of fluid containing fetal cells. This fluid contains fetal DNA, proteins like lecithin and sphingomyelin, which are used to assess lung maturity, with a lecithin/sphingomyelin (L/S) ratio above 2.0 indicating mature lungs. It also helps diagnose genetic disorders and neural tube defects.
Rationale for correct answers
C. Amniocentesis samples amniotic fluid to analyze fetal cells for chromosomal abnormalities (e.g., trisomy 21), genetic mutations, and biochemical markers for lung maturity such as the L/S ratio, providing critical information for fetal health assessment.
Rationale for incorrect answers
A. Measuring fundal height is a non-invasive clinical method to estimate fetal growth and weight, unrelated to amniocentesis.
B. Doppler ultrasound assesses uteroplacental blood flow and fetal circulation but does not involve sampling amniotic fluid.
D. Fetal heart tones are detected by Doppler or fetoscope, especially in early pregnancy, and are not confirmed by amniocentesis.
Take home points
- Amniocentesis is used to sample amniotic fluid for genetic and lung maturity testing.
- It provides critical prenatal diagnostic information on chromosomal abnormalities and fetal well-being.
- Fundal height measurement and Doppler ultrasound serve different diagnostic roles unrelated to amniocentesis.
- Fetal heart tones are confirmed by non-invasive methods, not amniocentesis.
Which of the following molecules primarily mediate blastocyst adhesion?
Explanation
Blastocyst adhesion is a critical step in implantation where the blastocyst attaches to the endometrial lining. This process is mediated by cell adhesion molecules such as integrins and cadherins, which facilitate specific binding between trophoblast cells and the extracellular matrix of the uterine epithelium. Integrins are transmembrane receptors that recognize extracellular matrix proteins like fibronectin, while cadherins mediate calcium-dependent cell-cell adhesion. This interaction occurs around day 6-7 post-fertilization and is essential for successful implantation and establishment of pregnancy.
Rationale for correct answers
C. Integrins and cadherins mediate blastocyst adhesion by promoting specific binding between the trophoblast and uterine epithelium. These molecules regulate the attachment and communication necessary for the blastocyst to invade and implant properly.
Rationale for incorrect answers
A. Estrogen and luteinizing hormone are endocrine hormones involved in ovulation and endometrial preparation but do not directly mediate cellular adhesion during blastocyst attachment.
B. Fibrin and collagen are extracellular matrix components but are not the primary molecules facilitating blastocyst adhesion; rather, they form structural scaffolds.
D. Histamine and prostaglandins are involved in inflammatory responses and uterine contractions but do not mediate the adhesive interactions of the blastocyst.
Take home points
- Integrins and cadherins are the key adhesion molecules enabling blastocyst attachment to the endometrium.
- Hormones like estrogen prepare the endometrium but do not directly mediate adhesion.
- Extracellular matrix proteins support tissue structure but are not primary adhesion mediators.
- Implantation requires coordinated molecular and cellular interactions for successful pregnancy.
Which of the following functions of amniotic fluid are critical for fetal lung development? Select all that apply
Explanation
Amniotic fluid plays an essential role in fetal development, particularly in the respiratory system. It provides a fluid medium that fills the fetal lungs, necessary for lung tissue expansion and maturation. The fetus practices breathing movements by inhaling and exhaling amniotic fluid, which stimulates the growth and differentiation of the pulmonary alveoli. Normal amniotic fluid volume ranges from 500 to 1000 mL in the third trimester, and abnormalities in volume can impair lung development, leading to conditions like pulmonary hypoplasia.
Rationale for correct answers
B. Amniotic fluid allows the fetus to perform breathing movements, critical for stimulating lung expansion and promoting structural and functional maturation of the lungs.
E. It provides the necessary fluid medium inside the lungs, which maintains lung distension and facilitates alveolar growth, essential for postnatal respiratory function.
Rationale for incorrect answers
A. Acting as a mechanical buffer protects the fetus from external trauma but does not directly affect lung development.
C. While amniotic fluid supports symmetrical growth of the musculoskeletal system, this is unrelated to pulmonary maturation.
D. Prevention of umbilical cord compression ensures adequate blood flow and oxygen delivery but is not directly linked to lung development.
Take home points
- Amniotic fluid is essential for fetal lung growth by providing a fluid medium within the lungs.
- Fetal breathing movements in amniotic fluid stimulate pulmonary development.
- Mechanical protection and musculoskeletal support are important but not lung-specific functions.
- Adequate amniotic fluid volume is crucial to prevent pulmonary hypoplasia.
Which of the following are the primary functions of the placenta? Select all that apply
Explanation
The placenta is a vital organ for fetal development, facilitating maternal-fetal exchange. It performs gas exchange, nutrient delivery, and waste removal between maternal and fetal blood without direct blood mixing. The placenta also transfers immunoglobulins, mainly immunoglobulin G (IgG), providing passive immunity to the fetus. It produces hormones essential for pregnancy maintenance but does not produce fetal hemoglobin or regulate fetal blood pressure directly. Normal placental weight ranges from 400 to 600 grams at term.
Rationale for correct answers
A. The placenta facilitates the transfer of oxygen from maternal blood to fetal blood via diffusion through the placental membrane, critical for fetal oxygenation.
B. It acts as an interface for the excretion of fetal waste products such as carbon dioxide and urea into maternal circulation for elimination.
C. The placenta transfers maternal immunoglobulins, especially IgG, across the syncytiotrophoblast to the fetal circulation, providing passive immunity.
Rationale for incorrect answers
D. Fetal hemoglobin (HbF) is synthesized by the fetal liver and bone marrow, not by the placenta.
E. Regulation of fetal blood pressure is controlled by fetal cardiovascular and renal systems, not directly by the placenta.
Take home points
- The placenta transfers oxygen and nutrients and removes fetal wastes.
- It mediates passive immunity by transferring maternal antibodies to the fetus.
- Placenta does not synthesize fetal hemoglobin or regulate fetal blood pressure.
- Placental function is essential for fetal survival and growth.
Which of the following conditions must be met for fertilization to occur? Select all that apply
Explanation
Fertilization is the union of a mature sperm and ovum resulting in a zygote. Successful fertilization depends on sperm capacitation, which is the biochemical modification of sperm in the female reproductive tract that enables penetration of the ovum. Sperm must exhibit adequate motility to traverse the cervical mucus, uterus, and fallopian tubes. The cervical mucus undergoes cyclical changes, becoming thin and less viscous during ovulation to facilitate sperm passage. The ovum’s viability is typically 12 to 24 hours post-ovulation, not 48 hours, and sperm are usually deposited in the vagina, not directly in the uterus.
Rationale for correct answers
B. Capacitation involves the removal of glycoproteins and seminal plasma proteins from sperm, which enables acrosomal reaction necessary for ovum penetration.
D. Sperm motility is essential for migration through the cervix, uterus, and fallopian tubes, overcoming the challenges of the female reproductive environment.
E. Thin, less viscous cervical mucus during ovulation reduces resistance, allowing sperm to swim effectively toward the ovum.
Rationale for incorrect answers
A. Sperm are normally deposited in the vagina during coitus, not directly in the uterus; uterine deposition is not a prerequisite for fertilization.
C. The ovum remains viable for approximately 12 to 24 hours after ovulation; viability for 48 hours is incorrect, as the fertilization window is shorter.
Take home points
- Capacitation is necessary to activate sperm for fertilization.
- Adequate sperm motility is crucial for successful sperm migration.
- Cervical mucus consistency changes to facilitate sperm passage during ovulation.
- Ovum viability after ovulation is limited to about 12 to 24 hours.
The three regions of the decidua include which of the following? Select all that apply
Explanation
The decidua is the modified endometrial lining of the uterus during pregnancy, essential for implantation and support of the developing embryo. It comprises three distinct regions based on their relation to the implanted embryo: the decidua basalis (beneath the implantation site), the decidua capsularis (surrounding the embryo), and the decidua parietalis (lining the remaining uterine cavity). These layers facilitate maternal-fetal interface, immune modulation, and vascular adaptation necessary for fetal development. Normal thickness of the decidua varies but is generally 5 to 7 mm during early pregnancy.
Rationale for correct answers
A. The decidua basalis is the region of the endometrium directly beneath the implanted blastocyst and forms the maternal part of the placenta, crucial for nutrient exchange and trophoblast invasion.
B. The decidua capsularis surrounds the implanted embryo, enclosing it within the uterine cavity before it eventually fuses with the decidua parietalis as the pregnancy progresses.
C. The decidua parietalis lines the remainder of the uterine cavity not involved in implantation and eventually fuses with the decidua capsularis by mid-pregnancy.
Rationale for incorrect answers
D. Decidua chorion is not a recognized anatomical subdivision of the decidua; the chorion is a separate fetal membrane derived from trophoblast cells.
E. Decidua embryonalis is not a standard anatomical term for decidual regions; the term is sometimes mistakenly used but does not correspond to any specific decidual layer.
Take home points
- The decidua has three regions: basalis, capsularis, and parietalis.
- Decidua basalis forms the maternal portion of the placenta.
- Decidua capsularis encloses the embryo initially and later fuses with the parietalis.
- Proper decidual development is critical for implantation and pregnancy maintenance.
Fetal growth restriction (FGR). can be associated with oligohydramnios. The nurse understands that this link is most commonly due to which of the following? Select all that apply
Explanation
Fetal growth restriction (FGR) is a condition where the fetus fails to achieve its genetically predetermined growth potential, often due to placental insufficiency, leading to chronic hypoxia and nutrient deprivation. Oligohydramnios, defined as an amniotic fluid index less than 5 cm or a single deepest pocket less than 2 cm, frequently accompanies FGR. Amniotic fluid volume depends primarily on fetal urine production, lung fluid secretion, and swallowing. Placental dysfunction, fetal renal anomalies, and altered fetal circulation can decrease urine output, reducing amniotic fluid and causing oligohydramnios.
Rationale for correct answers
A. Reduced fetal urine output occurs secondary to placental insufficiency, which causes hypoxia and redistribution of blood flow, limiting kidney perfusion and thus decreasing urine production—the major source of amniotic fluid in the third trimester.
C. Congenital anomalies of the fetal renal system impair urine formation or excretion, directly reducing amniotic fluid volume, as fetal urine constitutes up to 90% of amniotic fluid after 16 weeks gestation.
E. A shift in fetal blood flow away from the kidneys (known as blood flow redistribution or “brain-sparing”) prioritizes vital organs during hypoxia, reducing renal perfusion and urine output, thus contributing to oligohydramnios.
Rationale for incorrect answers
B. Increased fetal swallowing of amniotic fluid is not a typical compensatory mechanism in FGR; rather, swallowing usually maintains normal fluid balance and is not significantly increased in FGR or oligohydramnios.
D. Absence of functional kidneys (e.g., bilateral renal agenesis) leads to anuria and severe oligohydramnios, but this is a specific congenital anomaly rather than a common cause of FGR-related oligohydramnios.
Take home points
- Placental insufficiency decreases fetal renal perfusion, reducing urine output and amniotic fluid.
- Fetal renal congenital anomalies can impair urine production, leading to oligohydramnios.
- Fetal blood flow redistribution prioritizes brain over kidneys, reducing renal function.
- Oligohydramnios is a frequent finding in FGR and indicates compromised fetal well-being.
Exams on Fertilization and Implantation, Fetal Environment
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
Notes Highlighting is available once you sign in. Login Here.
Objectives
- To describe the physiological processes of fertilization, including sperm and ovum transport, and the events leading to zygote formation.
- To explain the stages of early embryonic development from cleavage to blastocyst formation and the intricate process of implantation into the uterine wall.
- To identify the key components of the fetal environment, specifically the amnion, chorion, amniotic fluid, and placenta.
- To elucidate the structural characteristics and essential functions of the amnion, chorion, and amniotic fluid in supporting fetal growth and protection.
- To detail the comprehensive structure, development, and multifaceted functions of the placenta, emphasizing its critical roles in endocrine regulation, metabolism, and substance exchange between mother and fetus.
- To recognize potential deviations from normal amniotic fluid volume and placental variations that may impact maternal and fetal well-being.
Introduction
The journey from conception to the development of a viable fetus is an extraordinary biological marvel, orchestrated by a series of precise and coordinated cellular and molecular events. This intricate process commences with fertilization, the union of male and female gametes, followed by the crucial phase of implantation, where the nascent embryo establishes a vital connection with the maternal endometrium. Concurrently, a specialized fetal environment begins to form, encompassing the amnion, chorion, amniotic fluid, and the placenta. These structures collectively provide the necessary support, protection, and sustenance for the developing fetus throughout gestation. A profound understanding of these foundational maternal-newborn topics is paramount for nursing students, as it forms the basis for comprehending normal pregnancy progression, identifying potential complications, and delivering comprehensive, evidence-based care to both mother and child.
Fertilization
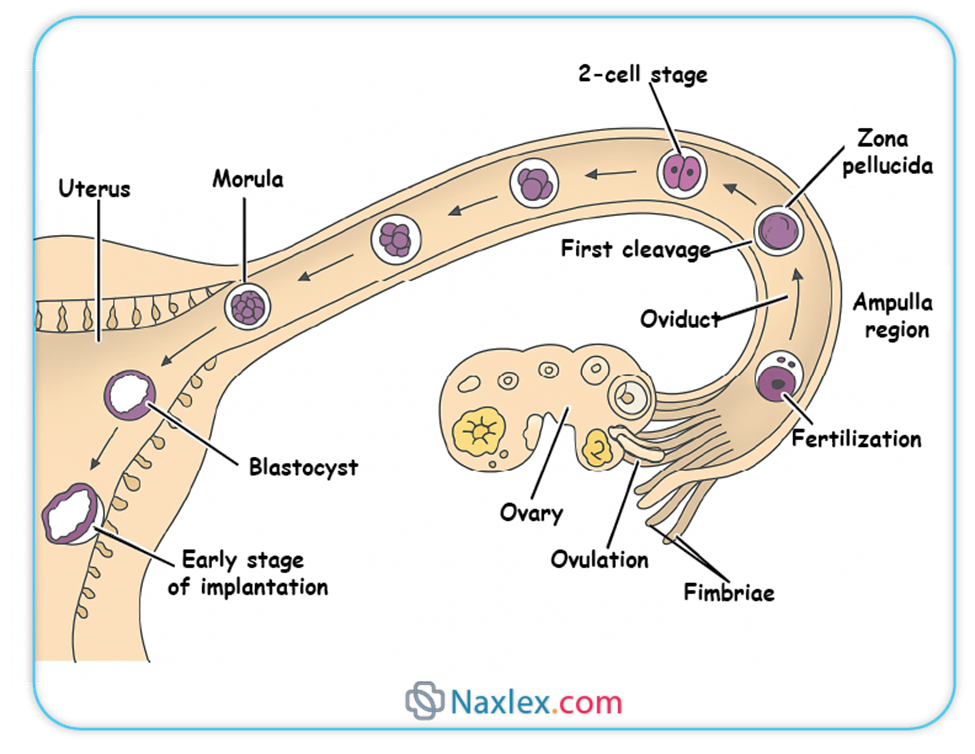
Fertilization is the fusion of male and female gametes (spermatozoon and ovum) to form a new individual, the zygote. This remarkable event typically occurs in the ampulla of the fallopian tube.
1. Sperm Transport and Capacitation
Spermatozoa are deposited in the vagina during coitus and must navigate a challenging journey through the cervix, uterus, and fallopian tubes to reach the ovum.
- Vaginal and Cervical Passage:
- The acidic environment of the vagina is hostile to sperm, with many being immobilized or killed.
- Cervical mucus, particularly around ovulation, becomes less viscous and forms channels, facilitating sperm passage into the uterus.
- Some sperm are stored in cervical crypts and released over several hours, contributing to sustained fertility.
- Uterine and Tubal Transport:
- Uterine contractions and the inherent motility of sperm aid their ascent through the uterine cavity and into the fallopian tubes.
- Only a small fraction of ejaculated sperm, typically a few hundred, reach the ampulla.
- Capacitation:
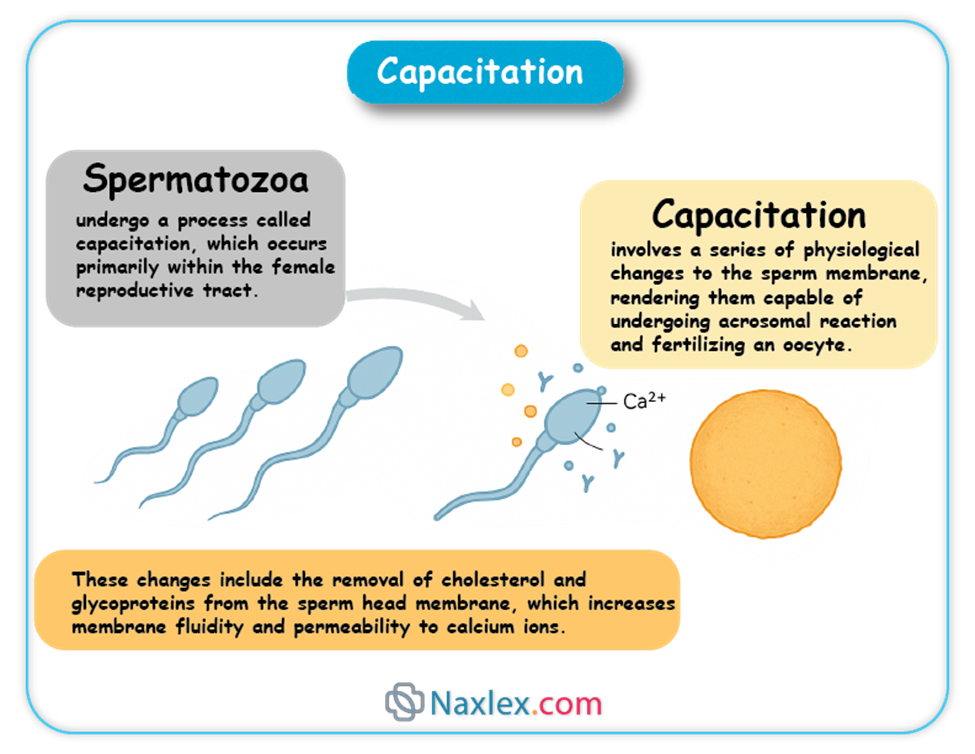
- Spermatozoa undergo a process called capacitation, which occurs primarily within the female reproductive tract.
- Capacitation involves a series of physiological changes to the sperm membrane, rendering them capable of undergoing the acrosomal reaction and fertilizing an oocyte.
- These changes include the removal of cholesterol and glycoproteins from the sperm head membrane, which increases membrane fluidity and permeability to calcium ions.
2. Ovum Transport
Ovulation typically releases a secondary oocyte, arrested in metaphase II of meiosis, from the graafian follicle.
- Fimbrial Capture:
- The fimbriae, finger-like projections at the end of the fallopian tube, sweep over the surface of the ovary and, through ciliary action, capture the released oocyte.
- Tubal Peristalsis and Ciliary Action:
- Once inside the fallopian tube, the oocyte is propelled towards the uterus by the rhythmic contractions (peristalsis) of the smooth muscle walls of the tube and the coordinated beating of cilia lining the tubal epithelium.
- Viability:
- The oocyte remains viable for fertilization for approximately 12-24 hours after ovulation.
3. The Process of Fertilization
Once capacitated sperm encounter the ovum, a series of events leads to the fusion of their genetic material.
3.1. Acrosomal Reaction
- Upon binding to the zona pellucida, the outer glycoprotein layer surrounding the ovum, capacitated sperm undergo the acrosomal reaction.
- This reaction involves the fusion of the outer acrosomal membrane with the sperm plasma membrane, leading to the release of hydrolytic enzymes, including hyaluronidase and acrosin.
- These enzymes are crucial for digesting the cumulus oophorus (granulosa cells surrounding the ovum) and creating a path through the zona pellucida for the sperm to reach the oocyte membrane.
3.2. Cortical Reaction (Block to Polyspermy)
- As soon as the first sperm makes contact with the oocyte plasma membrane, a rapid depolarization of the oocyte membrane occurs, known as the "fast block to polyspermy." This transient electrical change prevents immediate fusion by other sperm.
- Simultaneously, a more permanent "slow block to polyspermy" is initiated, called the cortical reaction.
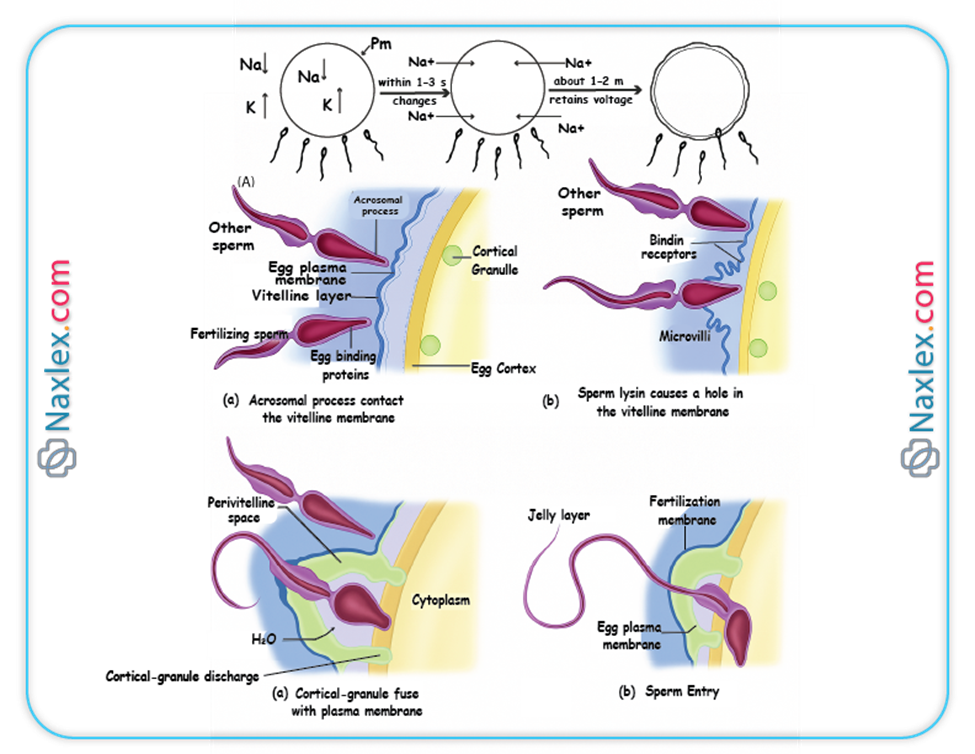
- The cortical reaction involves the release of cortical granules (lysosome-like vesicles located just beneath the oocyte membrane) into the perivitelline space (the space between the oocyte membrane and the zona pellucida).
- The enzymes released from these granules alter the structure of the zona pellucida (zona reaction), hardening it and inactivating sperm receptors, thereby preventing additional sperm from penetrating the oocyte. This ensures monospermy (fertilization by only one sperm).
3.3. Formation of the Zygote
- Once a single sperm enters the oocyte cytoplasm, it triggers the completion of meiosis II by the secondary oocyte, resulting in a mature ovum and the second polar body.
- The nucleus of the sperm decondenses and forms the male pronucleus.
- The nucleus of the mature ovum forms the female pronucleus.
- Both pronuclei replicate their DNA, and then their nuclear envelopes break down, allowing the chromosomes to combine, forming a single diploid nucleus.
- The cell containing this new diploid nucleus is now called a zygote, marking the successful completion of fertilization.
Nursing Insights: The understanding of fertilization is critical for nurses, especially in reproductive health and infertility. Explaining the timing of ovulation and the importance of sperm viability aids in patient education regarding conception. Recognizing that polyspermy is a lethal condition for the embryo highlights the precision of natural biological processes.
Implantation
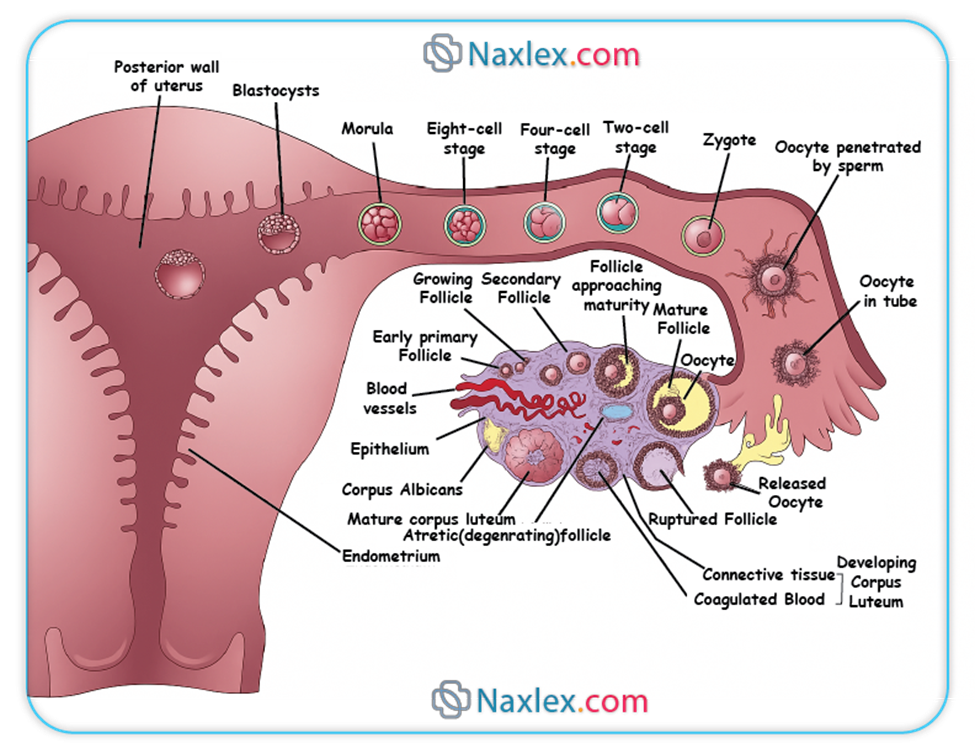
Implantation is the process by which the blastocyst, the early stage of embryonic development, attaches to and invades the maternal endometrium. This crucial event typically occurs around 6-10 days after fertilization.
1. Cleavage and Morula Formation
- Following fertilization, the zygote undergoes a series of rapid mitotic divisions called cleavage.
- These divisions occur without significant growth of the overall cell mass, meaning the daughter cells (blastomeres) become progressively smaller with each division.
- Day 1-2: The zygote divides into two blastomeres, then four.
- Day 3: The embryo consists of 8-16 blastomeres and is termed a morula (resembling a mulberry). The morula is still encased within the zona pellucida and is transported through the fallopian tube towards the uterus.
2. Blastocyst Formation
- As the morula enters the uterine cavity around day 4, fluid begins to penetrate the zona pellucida and accumulate within the intercellular spaces of the morula.
- This fluid coalesces to form a single fluid-filled cavity called the blastocoel (or blastocyst cavity).
- The cells of the morula differentiate into two distinct cell populations:
- Inner Cell Mass (ICM) or Embryoblast: A group of cells clustered at one pole of the blastocyst. These cells will give rise to the embryo proper and some extraembryonic membranes.
- Trophoblast: An outer layer of flattened cells surrounding the blastocoel and the inner cell mass. The trophoblast plays a crucial role in implantation and the formation of the placenta.
- At this stage, the embryo is called a blastocyst. Around day 5, the blastocyst "hatches" from the zona pellucida, allowing it to directly interact with the endometrial lining.
3. Process of Implantation
Implantation is a complex process involving several stages:
- Apposition:
- The blastocyst comes into close proximity with the endometrial lining. This is a transient and unstable adhesion.
- Adhesion (Attachment):
- The trophoblast cells of the blastocyst firmly attach to the endometrial epithelium.
- This attachment is mediated by various adhesion molecules, including integrins, selectins, and cadherins, expressed on both the trophoblast and endometrial cells.
- Invasion (Penetration):
- The trophoblast differentiates into two layers:
- Cytotrophoblast: The inner layer, composed of distinct cells with mitotic activity.
- Syncytiotrophoblast: The outer multinucleated layer formed by the fusion of cytotrophoblast cells. The syncytiotrophoblast lacks distinct cell boundaries and directly invades the maternal endometrium.
- The syncytiotrophoblast produces proteolytic enzymes (e.g., matrix metalloproteinases) that degrade the extracellular matrix of the endometrial tissue, allowing the blastocyst to burrow into the uterine wall.
- Lacunae (spaces) form within the syncytiotrophoblast, which will eventually fill with maternal blood, establishing the uteroplacental circulation.
- The trophoblast differentiates into two layers:
- Embedding:
- The blastocyst becomes completely embedded within the endometrial stroma, and the defect in the endometrial surface is sealed by a fibrin coagulum. This typically occurs by day 10-12 post-fertilization.
4. Decidua Formation
- In response to the invading blastocyst and hormonal influences (primarily progesterone), the endometrial stromal cells undergo a process called decidualization.
- Decidualization transforms the endometrial stromal cells into large, polyhedral decidual cells, rich in glycogen and lipids.
- The decidua provides an immunologically privileged environment for the developing embryo and plays a vital role in regulating trophoblast invasion and subsequent placental development.
- Three regions of the decidua are recognized:
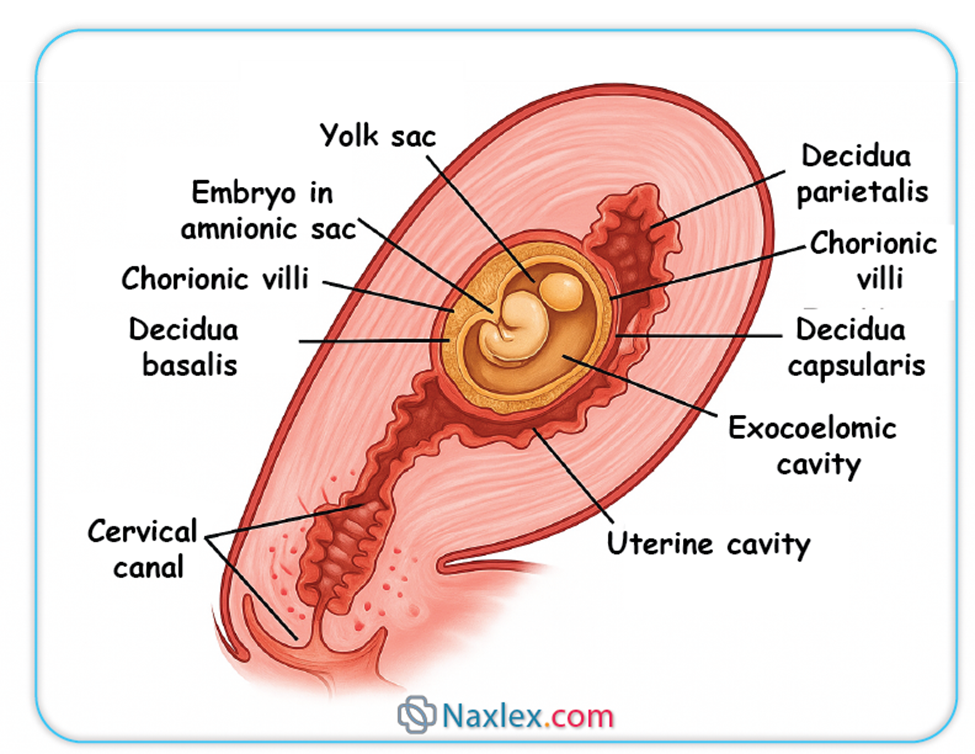
- Decidua Basalis: The portion of the decidua located beneath the implanted blastocyst, forming the maternal component of the placenta.
- Decidua Capsularis: The portion of the decidua overlying the implanted blastocyst, separating it from the uterine lumen. As the embryo grows, this layer becomes thin and eventually fuses with the decidua parietalis.
- Decidua Parietalis (Vera): The remaining part of the decidua lining the rest of the uterine cavity.
Nursing Insights: The timing and location of implantation are crucial. Ectopic pregnancies, where implantation occurs outside the uterus (most commonly in the fallopian tube), are a significant concern. Nurses must be aware of the signs and symptoms of ectopic pregnancy to ensure timely intervention, as it can be life-threatening. Understanding decidua formation also provides context for interpreting early pregnancy ultrasound findings.
Fetal Environment
The fetal environment refers to the structures that surround and support the developing fetus within the uterus. These include the amnion, chorion, amniotic fluid, and the placenta.
1. AMNION
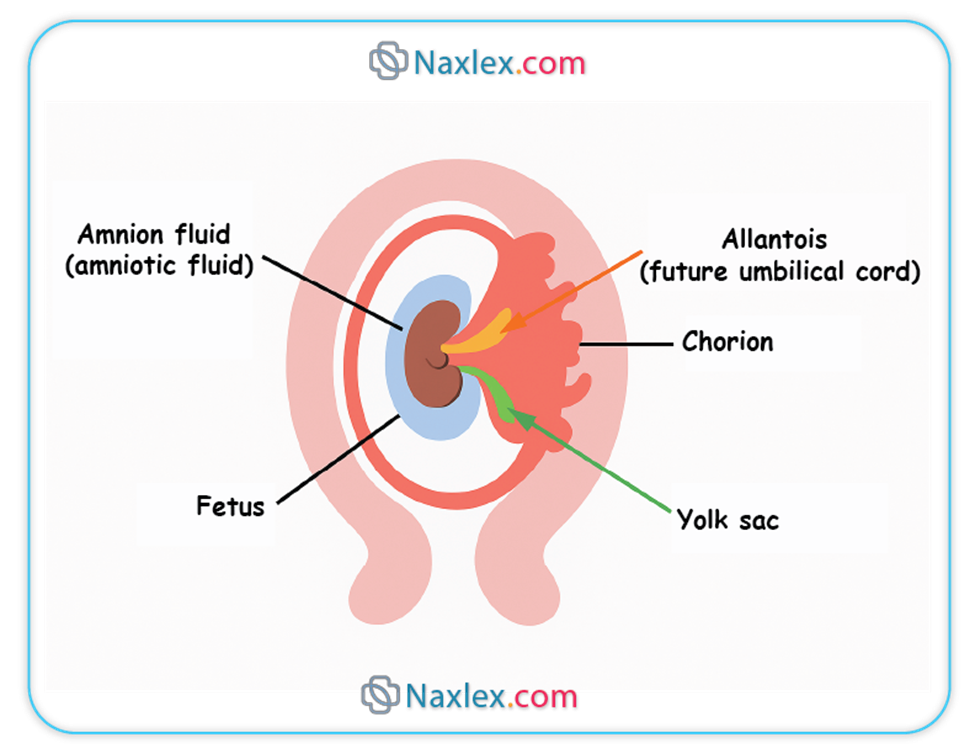
The amnion is the innermost fetal membrane, forming a fluid-filled sac that directly encloses the embryo and later the fetus.
1.1. Structure and Development
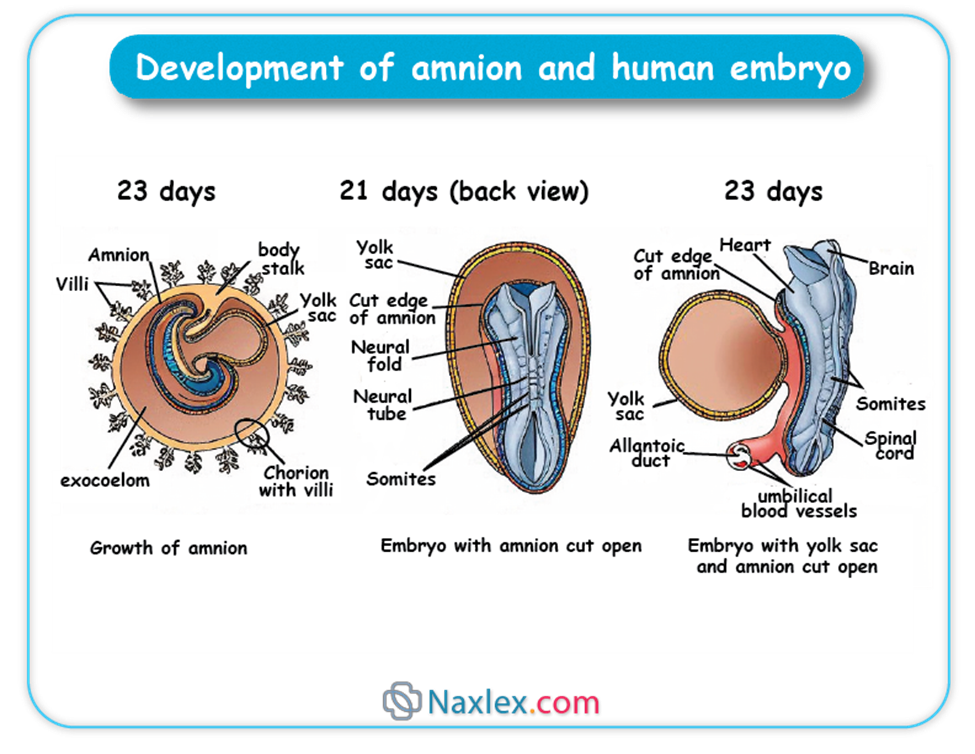
- The amnion develops from the epiblast during the second week of embryonic development, forming a thin, transparent membrane.
- Initially, the amnion is small and closely surrounds the embryonic disc.
- As the embryo grows and folds, the amnion expands rapidly to enclose the entire embryo, forming the amniotic cavity.
- The amniotic cavity eventually obliterates the chorionic cavity by fusing with the chorion, typically by the end of the first trimester.
- The amnion is composed of two layers:
- Amniotic Epithelium: An inner layer of simple cuboidal or columnar epithelial cells that face the amniotic fluid. These cells are responsible for secreting and absorbing amniotic fluid.
- Amniotic Mesoderm: An outer layer of connective tissue that is continuous with the chorion.
1.2. Functions of the Amnion
The amnion plays several vital roles in protecting and supporting the developing fetus:
- Formation of the Amniotic Cavity: It creates the space in which the fetus can grow and move freely.
- Production of Amniotic Fluid: The amniotic epithelial cells contribute to the secretion of amniotic fluid.
- Protection:
- Shock Absorber: The fluid-filled sac acts as a buffer against external mechanical trauma or pressure on the uterus.
- Temperature Regulation: It helps maintain a relatively constant temperature for the fetus.
- Prevents Adhesion: It prevents the amnion from adhering to the fetal skin, which could lead to congenital anomalies (amniotic band syndrome).
- Facilitates Fetal Movement and Growth:
- Allows for symmetrical musculoskeletal development by providing space for limb movement and preventing compression.
- Facilitates fetal swallowing and respiratory movements, contributing to the development of the gastrointestinal and respiratory systems, respectively.
Nursing Insights: Understanding the amnion's protective role helps nurses explain the importance of preventing abdominal trauma during pregnancy. Awareness of amniotic band syndrome, though rare, underscores the delicate nature of fetal development and the potential for mechanical forces to impact it.
2. CHORION
The chorion is the outermost fetal membrane, forming the protective sac that surrounds the amnion and the developing embryo/fetus.
2.1. Structure and Development
- The chorion develops from the trophoblast and extraembryonic mesoderm.
- It consists of two layers:
- Trophoblast: The outer layer, which further differentiates into cytotrophoblast and syncytiotrophoblast.
- Somatic Layer of Extraembryonic Mesoderm: An inner layer that lies beneath the trophoblast.
- Initially, the chorion is covered by chorionic villi all around its surface.
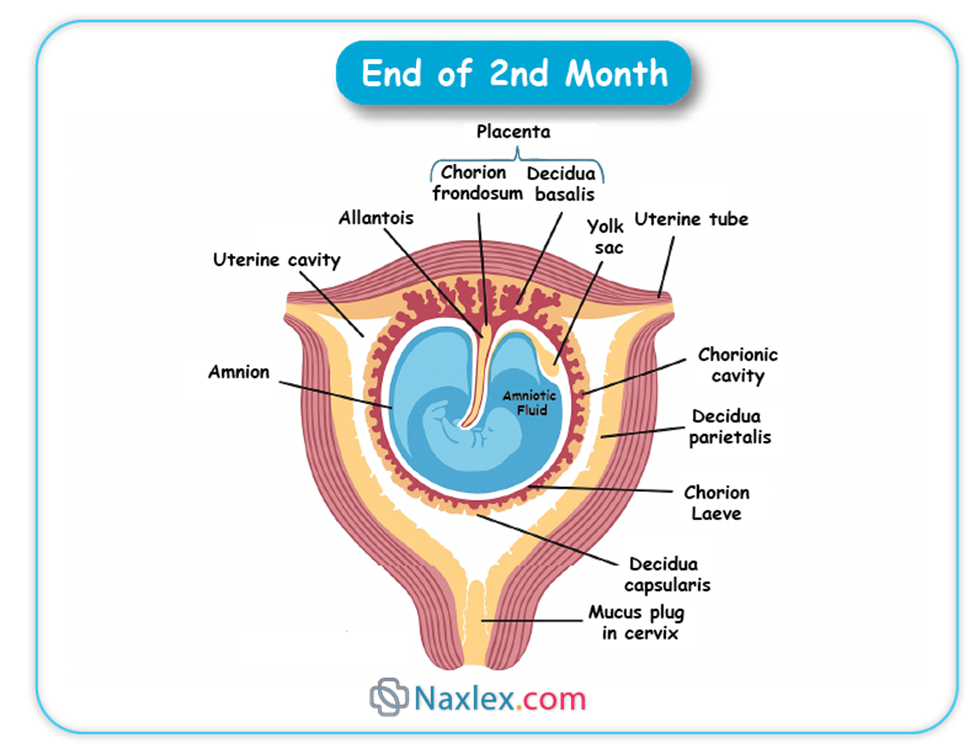
- As pregnancy progresses, the villi associated with the decidua capsularis degenerate, forming the smooth chorion (chorion laeve).
- The villi adjacent to the decidua basalis proliferate and branch extensively, forming the bushy chorion (chorion frondosum), which contributes to the fetal part of the placenta.
- The chorion encloses the amniotic sac, the yolk sac, and the connecting stalk.
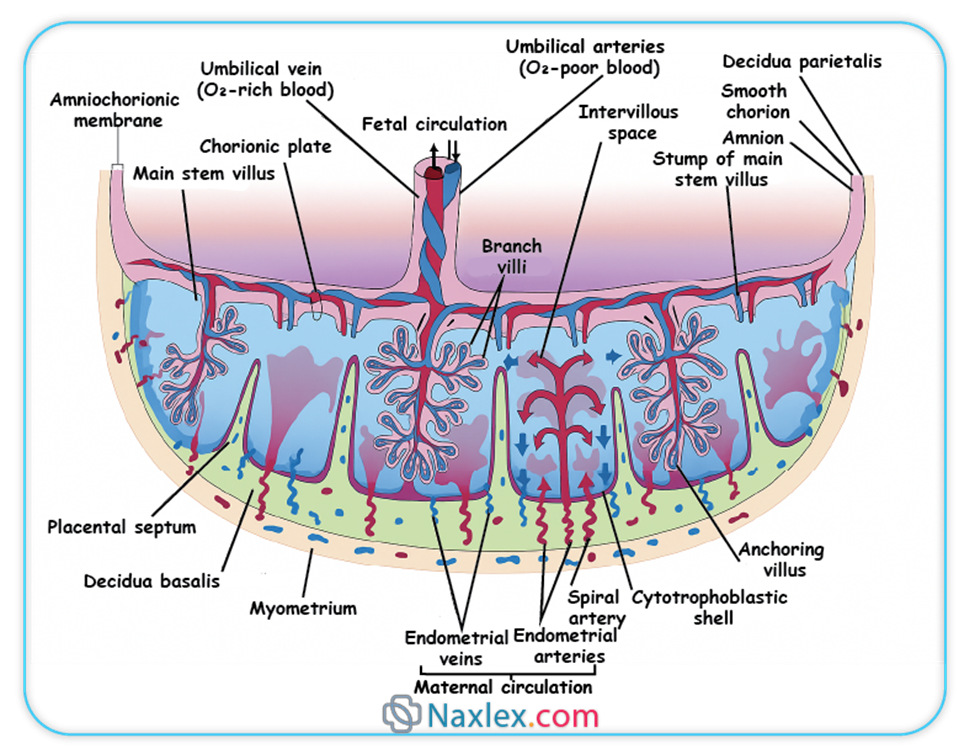
2.2. Functions of the Chorion
The chorion serves several critical functions:
- Formation of the Placenta: The chorion frondosum is the primary fetal component of the placenta.
- Exchange of Substances: Through the chorionic villi, it facilitates the exchange of nutrients, gases, and waste products between the maternal and fetal circulations.
- Hormone Production: The syncytiotrophoblast of the chorion produces various hormones essential for maintaining pregnancy, including human chorionic gonadotropin (hCG), progesterone, and estrogen.
- Protection: Along with the amnion, it forms a protective barrier around the fetus.
- Immunological Role: It helps to modulate the maternal immune response to prevent rejection of the fetal allograft.
Nursing Insights: The chorion's role in placenta formation is fundamental to understanding nutrient and waste exchange, which is directly relevant to fetal growth and well-being. The production of hCG by the chorion is the basis for most pregnancy tests, a key nursing assessment tool.
3. AMNIOTIC FLUID
Amniotic fluid is the clear, yellowish fluid that fills the amniotic sac, surrounding and protecting the developing fetus.
3.1. Origin and Composition
The origin and composition of amniotic fluid change throughout gestation:
- Early Pregnancy (First Trimester):
- Primarily derived from maternal plasma by diffusion across the amniotic membrane and fetal skin.
- A small amount is also contributed by secretion from the amniotic epithelial cells.
- Mid-to-Late Pregnancy (Second and Third Trimesters):
- Fetal Urine: Becomes the major contributor to amniotic fluid volume, starting around 10-12 weeks of gestation. The fetus swallows amniotic fluid, absorbs it into the bloodstream, and then excretes dilute urine into the amniotic cavity.
- Fetal Lung Fluid: Secretions from the fetal lungs also contribute to the fluid volume.
- Transudation Across Fetal Skin: Prior to keratinization (around 20-25 weeks), water and solutes can diffuse across the fetal skin. After keratinization, this contribution significantly decreases.
- Maternal Sources: Some fluid continues to transudate from the maternal circulation across the uteroplacental membranes.
- Composition:
- Primarily water (98-99%).
- Contains electrolytes, proteins, carbohydrates, lipids, urea, creatinine, hormones, enzymes, fetal cells (skin, genitourinary, gastrointestinal), vernix caseosa, and lanugo.
- The specific gravity is typically 1.008-1.010.
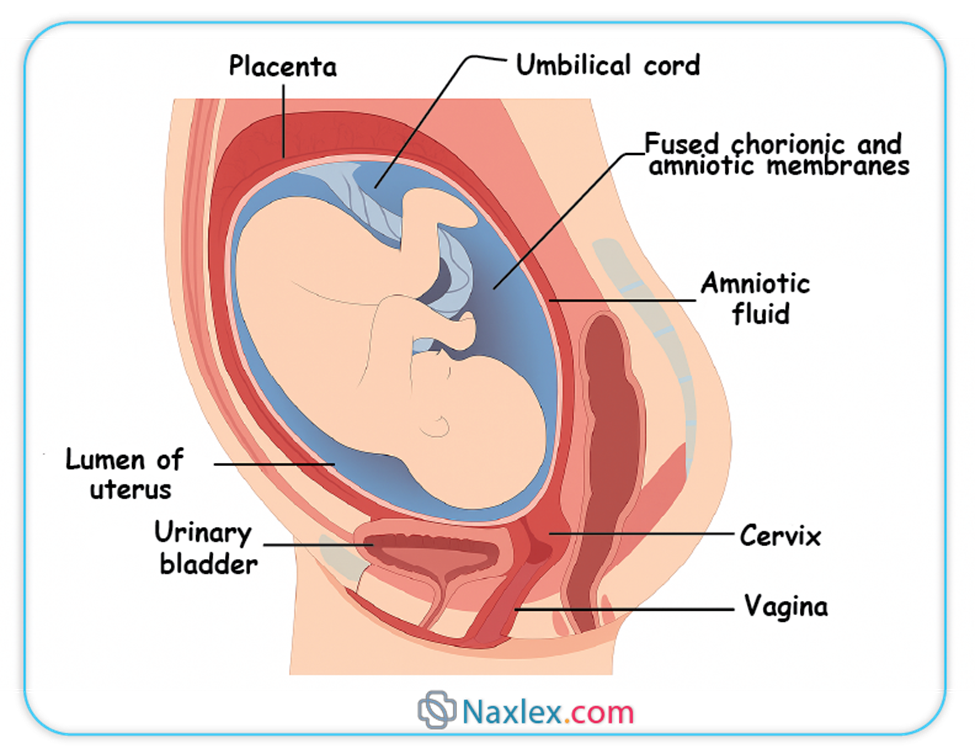
3.2. Functions of Amniotic Fluid
Amniotic fluid performs numerous vital functions for fetal development and protection:
- Protection from Trauma:
- Acts as a shock absorber, cushioning the fetus from external blows or pressure on the maternal abdomen.
- Temperature Regulation:
- Helps maintain a stable fetal body temperature, buffering against abrupt thermal changes.
- Musculoskeletal Development:
- Provides space for symmetrical growth and development of the fetal limbs and musculoskeletal system, allowing for freedom of movement.
- Prevents compression of the umbilical cord.
- Lung Development:
- Fetal breathing movements, involving the aspiration and expulsion of amniotic fluid, are essential for lung maturation and development of the pulmonary system.
- Gastrointestinal Development:
- Fetal swallowing of amniotic fluid contributes to the development and maturation of the gastrointestinal tract and aids in fluid and electrolyte balance.
- Prevents Adhesions:
- Keeps the fetal skin from adhering to the amnion.
- Antibacterial Properties:
- Contains some antibacterial substances that offer a minor degree of protection against infection.
- Diagnostic Tool:
- Amniocentesis (sampling of amniotic fluid) can be used to assess fetal chromosomal abnormalities, genetic disorders, and lung maturity.
3.3. Amniotic Fluid Volume
The volume of amniotic fluid fluctuates throughout pregnancy, typically increasing until 34-36 weeks gestation and then slightly decreasing. Normal volume is generally considered to be 800-1000 mL at term. Abnormalities in volume can indicate underlying fetal or maternal issues.
3.3.1. Polyhydramnios (Hydramnios)
- Definition: Excessive accumulation of amniotic fluid, typically defined as an amniotic fluid index (AFI) greater than 24-25 cm or a single deepest pocket (SDP) greater than 8 cm.
- Causes:
- Fetal Factors (most common):
- Fetal Dysphagia/Anencephaly: Inability of the fetus to swallow amniotic fluid (e.g., esophageal atresia, anencephaly, other CNS anomalies affecting swallowing reflex).
- Fetal Hydrops: Generalized edema in the fetus, leading to increased fluid accumulation.
- Chromosomal Abnormalities: Trisomy 18, 21.
- Fetal Tumors: Rarely, can obstruct swallowing or increase fluid production.
- Maternal Factors:
- Maternal Diabetes Mellitus: Poorly controlled maternal hyperglycemia can lead to fetal polyuria.
- Idiopathic: In many cases, no specific cause is identified.
- Placental Factors:
- Chorioangioma: A benign placental tumor that can lead to increased fluid production.
- Multiple Gestation: Especially in twin-twin transfusion syndrome.
- Fetal Factors (most common):
- Clinical Significance:
- Increased risk of preterm labor and premature rupture of membranes (PROM).
- Maternal discomfort (dyspnea, abdominal distension).
- Increased risk of postpartum hemorrhage due to uterine overdistension.
- Fetal malpresentation (breech, transverse lie).
- Umbilical cord prolapse upon rupture of membranes.
3.3.2. Oligohydramnios
- Definition: Insufficient amount of amniotic fluid, typically defined as an AFI less than 5 cm or an SDP less than 2 cm.
- Causes:
- Fetal Factors (most common):
- Renal Agenesis (Potter's Syndrome): Absence or severe underdevelopment of fetal kidneys, leading to absence or severely reduced urine production.
- Urinary Tract Obstruction: Blockage in the fetal urinary tract (e.g., posterior urethral valves), preventing urine excretion.
- Fetal Growth Restriction (FGR): Redistribution of blood flow away from non-essential organs, including the kidneys, in compromised fetuses.
- Premature Rupture of Membranes (PROM): Leakage of amniotic fluid.
- Maternal Factors:
- Dehydration.
- Placental Insufficiency: Reduced blood flow to the placenta, impacting fetal renal perfusion.
- Medications: Certain drugs, especially NSAIDs (e.g., indomethacin) used for preterm labor, can reduce fetal urine output.
- Post-term Pregnancy: Decreased placental function can lead to reduced fluid production.
- Fetal Factors (most common):
- Clinical Significance:
- Increased risk of fetal lung hypoplasia (underdevelopment of lungs) due to lack of fluid for breathing movements.
- Skeletal deformities (e.g., clubfoot, facial compression) due to uterine compression.
- Umbilical cord compression, leading to variable decelerations and fetal hypoxia.
- Fetal growth restriction.
- Increased risk of stillbirth.
Nursing Insights: Nurses play a crucial role in assessing amniotic fluid volume through physical examination (fundal height) and monitoring ultrasound reports. Explaining the implications of abnormal fluid volumes to expectant parents is essential, as is preparing them for potential interventions or complications associated with polyhydramnios or oligohydramnios.
Placenta
The placenta is a remarkable temporary organ that serves as the primary interface between the mother and the developing fetus, facilitating crucial exchange processes and hormone production essential for pregnancy maintenance.
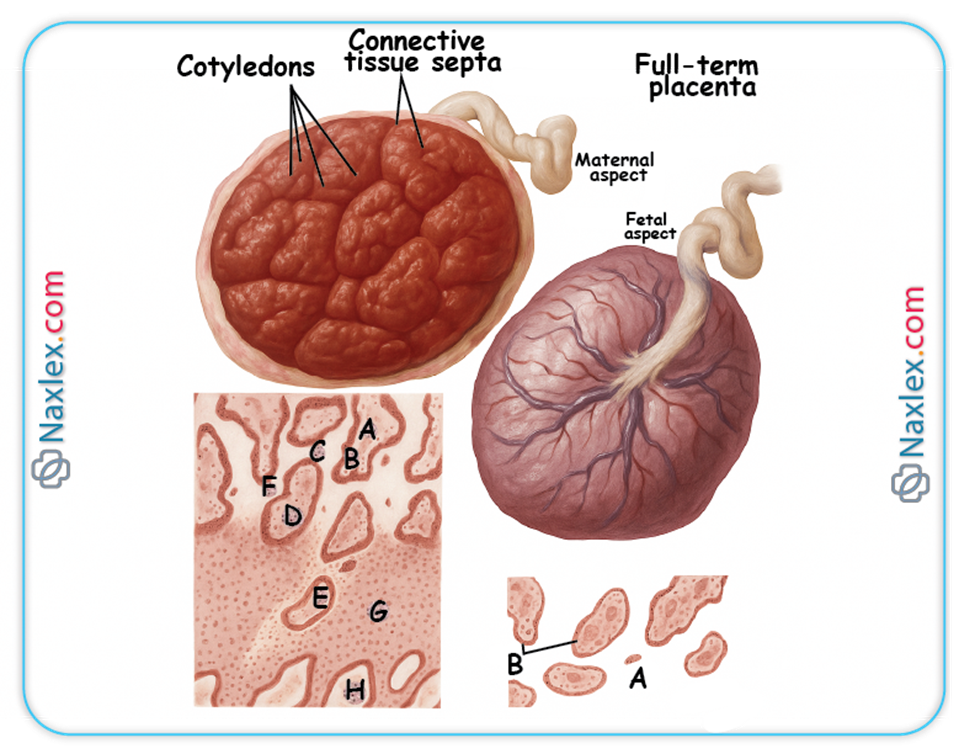
1. Structure and Development
- Development:
- Begins to form during implantation from the trophoblast of the blastocyst and the decidua basalis of the uterus.
- The chorion frondosum (bushy chorion) forms the fetal component, while the decidua basalis forms the maternal component.
- By the end of the first trimester (approximately 12 weeks), the placenta is fully formed and functional.
- Gross Structure at Term:
- Shape: Typically discoid or disc-shaped.
- Size: Approximately 15-20 cm in diameter, 2-3 cm thick at the center.
- Weight: Around 500-600 grams (about 1/6th of the fetal weight).
- Two Surfaces:
- Maternal Surface (Cotyledons): Appears rough and irregular, divided into 15-20 convex areas called cotyledons, which are formed by decidual septa. The cotyledons represent the lobes of the placenta, each containing a main stem villus and its branches.
- Fetal Surface (Shiny Schultze): Appears smooth and shiny, covered by the amnion. The umbilical cord typically inserts near the center, and blood vessels (umbilical arteries and vein) can be seen radiating from the cord insertion site.
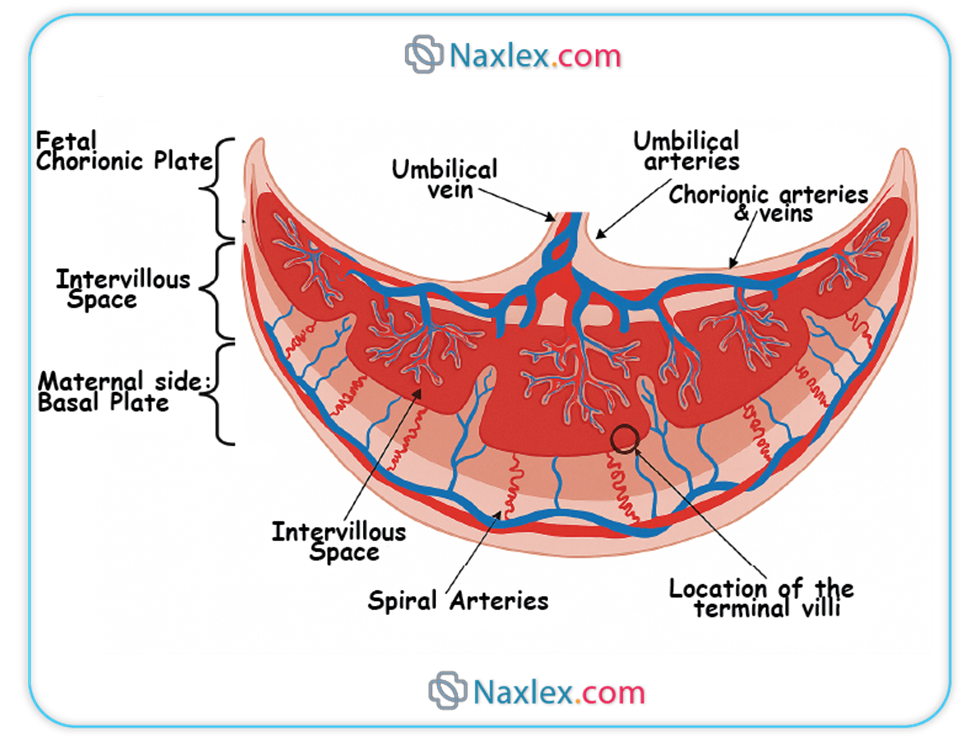
- Microscopic Structure (Placental Barrier):
- The functional unit of the placenta is the chorionic villus. These villi project into the intervillous space, which is filled with maternal blood.
- The "placental barrier" or "placental membrane" is a misnomer, as it is not an impermeable barrier. It represents the layers of tissue that separate maternal and fetal blood.
- In early pregnancy, this barrier is thicker, consisting of:
- Syncytiotrophoblast
- Cytotrophoblast
- Connective tissue of the villus (stroma)
- Endothelium of the fetal capillary
- As pregnancy progresses, the cytotrophoblast layer thins and largely disappears, and the syncytiotrophoblast thins significantly, bringing the fetal capillaries closer to the maternal blood in the intervillous space. This thinning facilitates more efficient exchange.
2. Functions of the Placenta
The placenta is a multifunctional organ critical for sustaining pregnancy.
2.1. Endocrine Functions
The syncytiotrophoblast is a major endocrine organ, producing numerous hormones essential for maintaining pregnancy:
- Human Chorionic Gonadotropin (hCG):
- Secreted shortly after implantation.
- Maintains the corpus luteum in the ovary during early pregnancy, ensuring continued production of progesterone and estrogen until the placenta is mature enough to take over.
- Is the hormone detected in pregnancy tests.
- Progesterone:
- Produced in increasing amounts by the placenta from approximately 7-8 weeks gestation, eventually becoming the primary source.
- Functions:
- Maintains the decidua.
- Relaxes uterine smooth muscle, preventing contractions and preterm labor.
- Suppresses maternal immune response to prevent rejection of the fetus.
- Inhibits synthesis of prostaglandins.
- Prepares mammary glands for lactation.
- Estrogens (primarily Estriol):
- Produced by the placenta using androgen precursors from the fetal adrenal glands and maternal cholesterol. This unique fetoplacental unit interaction is essential for estrogen synthesis.
- Functions:
- Stimulates uterine growth and uteroplacental blood flow.
- Contributes to the development of maternal mammary glands.
- Increases uterine contractility towards term.
- Human Placental Lactogen (hPL) / Human Chorionic Somatomammotropin (hCS):
- Produced by the syncytiotrophoblast.
- Functions:
- Modifies maternal metabolism to provide more nutrients for fetal growth (e.g., increases maternal insulin resistance, promotes lipolysis).
- Stimulates mammary gland development for lactation.
- Relaxin:
- Produced by the decidua and placenta.
- Relaxes pelvic ligaments and softens the cervix, preparing for labor.
2.2. Metabolic Functions
The placenta also performs various metabolic activities:
- Glycogen Synthesis: Stores glycogen, providing a readily available energy source for the fetus.
- Fatty Acid Synthesis: Synthesizes fatty acids, which are important for fetal growth and development.
- Protein Synthesis: Can synthesize some proteins.
- Nutrient Storage: Stores iron, glycogen, and fat-soluble vitamins.
2.3. Transfer Functions
The placenta facilitates the transfer of substances between the mother and fetus through various mechanisms:
2.3.1. Gas Exchange
- Oxygen and carbon dioxide are transferred across the placental membrane primarily by simple diffusion.
- Fetal hemoglobin has a higher affinity for oxygen than adult hemoglobin, facilitating oxygen uptake.
2.3.2. Nutrient Transfer
- Glucose: Transferred by facilitated diffusion via glucose transporters (GLUTs). It is the primary energy source for the fetus.
- Amino Acids: Transferred by active transport, leading to higher concentrations in fetal blood than maternal blood. Essential for fetal protein synthesis.
- Fatty Acids: Primarily transferred by simple diffusion, especially free fatty acids.
- Vitamins and Minerals: Transferred by various mechanisms, including active transport (e.g., iron, calcium, folate) and facilitated diffusion.
2.3.3. Waste Product Excretion
- Urea, Creatinine, Uric Acid: Transferred from fetal to maternal blood by simple diffusion for excretion by the maternal kidneys.
2.3.4. Antibody Transfer
- Immunoglobulin G (IgG): Actively transported across the placenta, primarily in the third trimester.
- Provides passive immunity to the fetus against various maternal infections (e.g., measles, rubella, tetanus, diphtheria), offering protection during the first few months of life. Other immunoglobulins (IgA, IgM) do not cross the placenta significantly.
2.3.5. Drug Transfer
- Most drugs with a molecular weight less than 1000 Da can cross the placenta.
- Transfer depends on factors like lipid solubility, molecular size, protein binding, and concentration gradient.
- This is a critical concern as many medications and illicit substances can have teratogenic or adverse effects on the fetus.
Nursing Insights: The placenta's role as a major endocrine organ explains the hormonal changes observed in pregnancy. Its transfer functions underscore the importance of maternal nutrition and avoidance of harmful substances. The transfer of maternal antibodies highlights the natural protection afforded to newborns, influencing vaccination schedules and disease susceptibility.
3. Placental Circulation
The placenta serves as the interface for two separate circulatory systems: maternal and fetal.
- Maternal Placental Circulation:
- Maternal blood enters the intervillous space from the uterine arteries, which are branches of the internal iliac arteries.
- These spiral arteries pierce the decidua basalis and open directly into the intervillous space.
- Maternal blood then bathes the chorionic villi, facilitating exchange.
- Deoxygenated and waste-laden blood drains from the intervillous space through the endometrial veins back into the maternal systemic circulation.
- Crucially, there is no direct mixing of maternal and fetal blood under normal physiological conditions.
- Fetal Placental Circulation:
- Deoxygenated blood and waste products are carried from the fetus to the placenta via two umbilical arteries (branches of the fetal internal iliac arteries).
- These arteries enter the placenta, branch extensively, and supply the capillary networks within the chorionic villi.
- Within the villi, exchange of gases, nutrients, and waste products occurs between fetal capillary blood and maternal blood in the intervillous space.
- Oxygenated and nutrient-rich blood is returned to the fetus via a single umbilical vein.
- The umbilical cord typically contains two umbilical arteries and one umbilical vein, embedded in Wharton's jelly (a gelatinous connective tissue that protects the vessels).
Nursing Insights: Understanding placental circulation is fundamental to interpreting fetal monitoring (e.g., non-stress tests, biophysical profiles) and recognizing signs of placental insufficiency, which can lead to fetal hypoxia and growth restriction. The presence of two arteries and one vein in the umbilical cord is a normal finding, and deviations (e.g., single umbilical artery) warrant further investigation for potential fetal anomalies.
4. Variations and Abnormalities of the Placenta
Variations in placental structure and position can affect pregnancy outcomes:
- Placenta Previa:
- Definition: Implantation of the placenta over or near the internal os of the cervix.
- Types: Complete/Total (covers the entire os), Partial (partially covers), Marginal (edge reaches the os), Low-lying (near but not covering).
- Clinical Significance: Painless vaginal bleeding in the second or third trimester, increased risk of hemorrhage, preterm birth, need for Cesarean section.
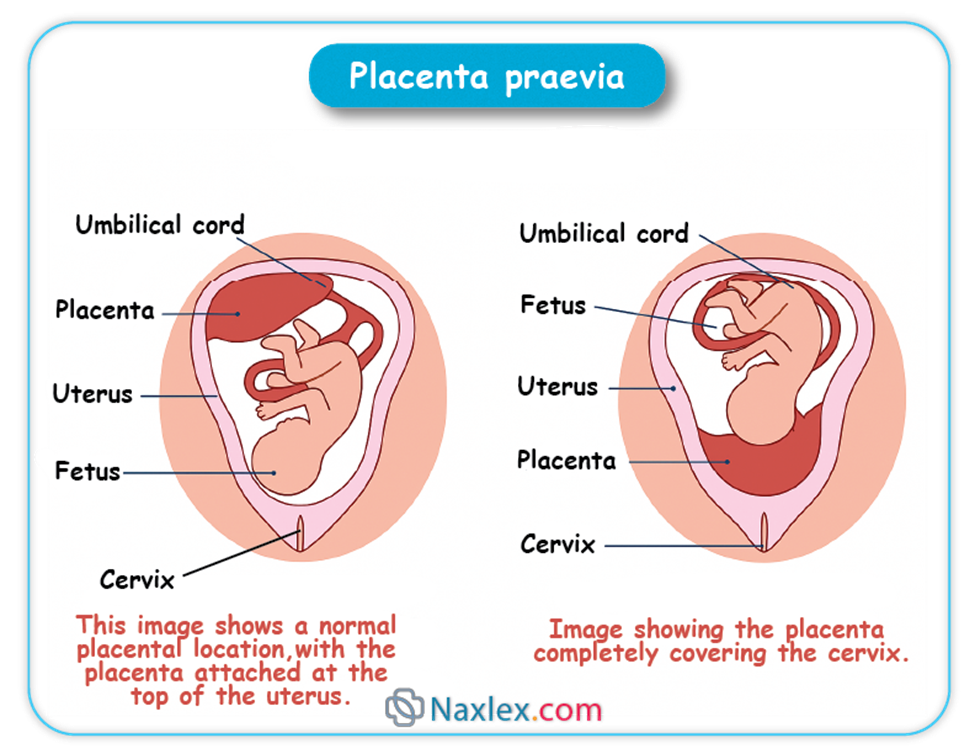
- Placenta Accreta/Increta/Percreta:
- Definition: Abnormal adherence of the placenta to the uterine wall due to defective decidua basalis.
- Accreta: Villi attach to the myometrium.
- Increta: Villi invade the myometrium.
- Percreta: Villi penetrate through the myometrium and possibly into adjacent organs (e.g., bladder).
- Clinical Significance: Severe postpartum hemorrhage, often requiring hysterectomy. Risk factors include previous Cesarean sections, placenta previa, and uterine surgery.
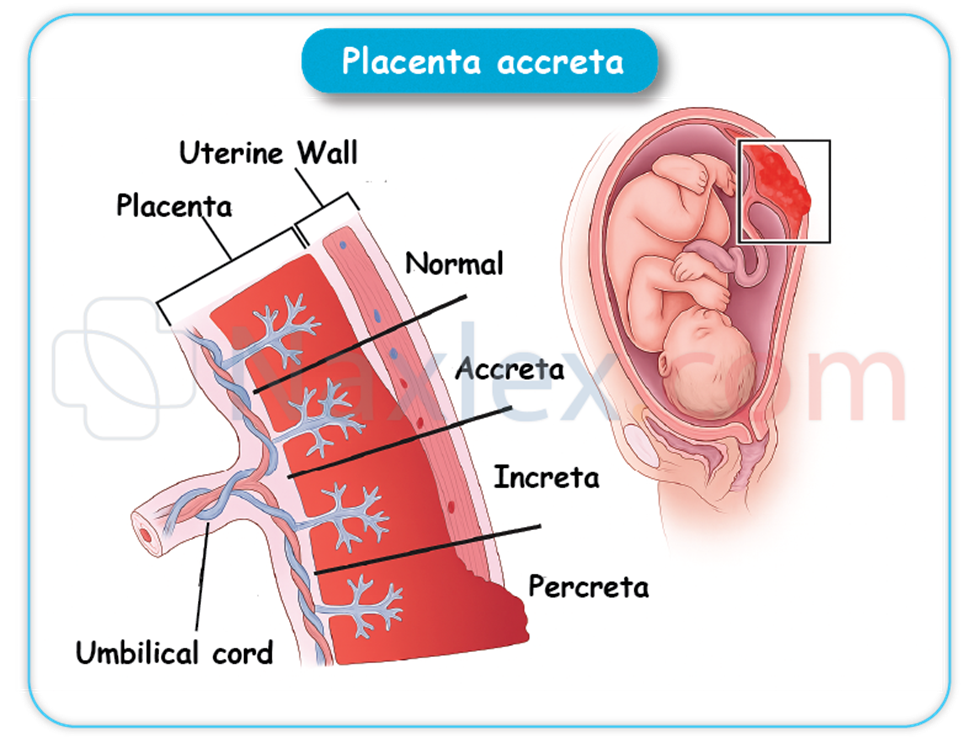
- Vasa Previa:
- Definition: Fetal blood vessels (umbilical vessels) traverse the membranes unprotected by placental tissue or umbilical cord, lying in front of the presenting part.
- Clinical Significance: High risk of fetal exsanguination (hemorrhage) if membranes rupture and vessels are compressed or torn, particularly during labor.
- Battledore Placenta (Marginal Cord Insertion):
- The umbilical cord inserts at the margin or periphery of the placenta rather than centrally. Usually of no clinical significance unless the insertion site is compromised.
- Velamentous Cord Insertion:
- Umbilical vessels separate in the membranes some distance from the placental margin and then travel within the membranes to insert into the placenta.
- Increases risk of vasa previa and vessel compression/rupture.
- Succenturiate Lobe:
- One or more accessory lobes of placental tissue are connected to the main placenta by blood vessels.
- Risk of a retained succenturiate lobe after delivery, leading to postpartum hemorrhage or infection.
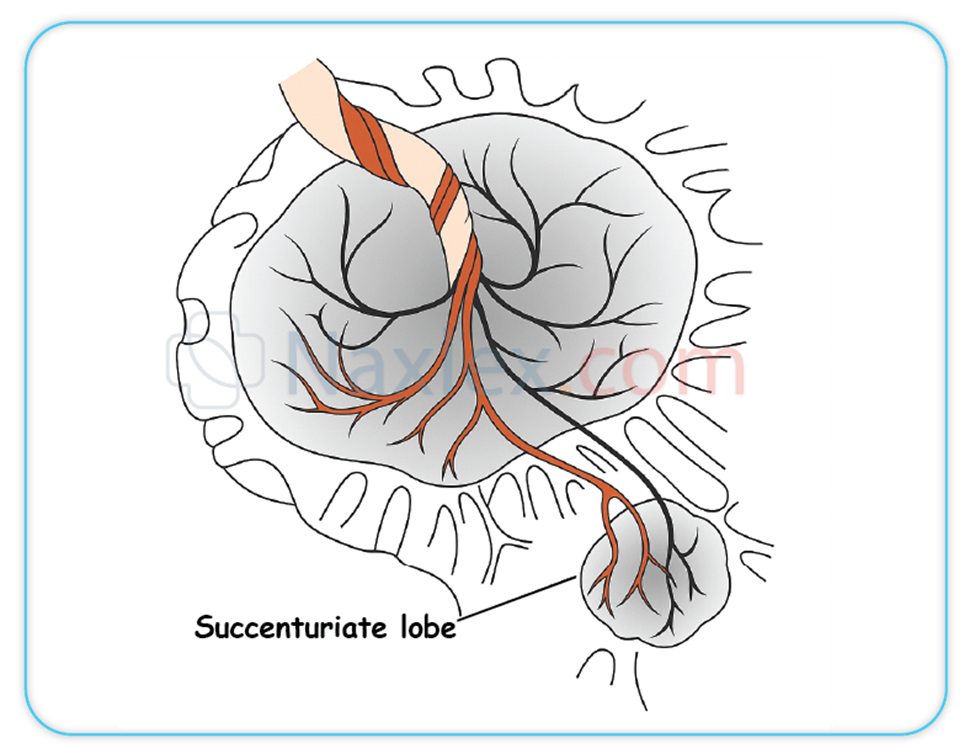
- Circumvallate Placenta:
- A double fold of amnion and chorion forms a ring around the periphery of the placenta on the fetal surface, with vessels extending beyond this ring.
- Associated with preterm birth, hemorrhage, and placental abruption.
Nursing Insights: Nurses must be vigilant for signs and symptoms of placental abnormalities, especially bleeding. Understanding these conditions informs patient education, prepares for potential emergencies (e.g., massive transfusion protocols for accreta), and ensures appropriate monitoring and interventions to optimize maternal and fetal outcomes.
Summary
The initiation and progression of human pregnancy are founded upon a series of remarkably coordinated biological events, beginning with the precise union of gametes. Fertilization, typically occurring in the fallopian tube, involves the intricate journey of sperm, their capacitation, and the subsequent penetration of the ovum, culminating in the formation of a diploid zygote. Following fertilization, the zygote undergoes rapid cleavage divisions to form a morula, which then transforms into a blastocyst with distinct inner cell mass and trophoblast components. This blastocyst subsequently undergoes implantation into the prepared decidua of the maternal endometrium, establishing the critical connection for pregnancy.
Concurrently with early embryonic development, the fetal environment begins to take shape. The amnion, the innermost membrane, forms the amniotic cavity, which fills with amniotic fluid. This fluid, primarily composed of fetal urine in later stages, serves numerous vital functions: providing mechanical protection, maintaining thermal stability, facilitating musculoskeletal and organ development (especially lungs and GI tract), and preventing adhesions. Abnormalities in amniotic fluid volume, such as polyhydramnios (excess) or oligohydramnios (deficient), indicate potential fetal or maternal complications. The chorion, the outermost fetal membrane, plays a crucial role in forming the fetal component of the placenta.
The placenta is a temporary yet indispensable organ, serving as the interface for maternal-fetal exchange. Structurally, it comprises both maternal (decidua basalis) and fetal (chorion frondosum) components. Functionally, the placenta acts as a multifaceted organ: performing crucial endocrine functions by producing hormones like hCG, progesterone, estrogens, and hPL to maintain pregnancy; facilitating essential metabolic functions for fetal growth; and mediating vital transfer functions of gases, nutrients, waste products, and maternal antibodies between the two circulations. The unique placental circulation ensures efficient exchange without direct mixing of maternal and fetal blood. Variations and abnormalities of the placenta, such as placenta previa or accreta, carry significant risks and necessitate diligent nursing assessment and intervention. A comprehensive understanding of these interconnected processes is paramount for nurses to provide high-quality, evidence-based care throughout the maternal-newborn continuum.
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Fertilization and Implantation, Fetal Environment
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


