Please set your exam date
Fetal Circulation
Study Questions
Practice Exercise 1
What is the primary function of the placenta in fetal circulation?
Explanation
The placenta is a highly specialized, temporary organ that develops during pregnancy, serving as the crucial interface between the maternal and fetal circulations. This vital structure performs multiple essential functions to sustain fetal growth and development, including nutrient exchange, waste removal, and the production of various hormones necessary for maintaining pregnancy. It effectively acts as the fetus's lungs, kidneys, and gastrointestinal tract while these fetal organs are immature and non-functional.
Rationale for correct answers
B. The primary and most critical function of the placenta in fetal circulation is to facilitate the efficient exchange of gases, specifically oxygen from the maternal blood to the fetal blood and carbon dioxide from the fetal blood to the maternal blood. Concurrently, it ensures the robust delivery of essential nutrients, such as glucose, amino acids, vitamins, and minerals, from the mother to the developing fetus, while also removing metabolic waste products.
Rationale for incorrect answers
A. The production of fetal hemoglobin (HbF) occurs within the fetal hematopoietic system, primarily in the fetal liver during early gestation and subsequently in the bone marrow, not within the placenta. HbF is distinct from adult hemoglobin due to its higher affinity for oxygen, a crucial adaptation for oxygen transfer in the relatively hypoxic intrauterine environment.
C. Fetal heart rate regulation is primarily governed by the intrinsic rhythmicity of the fetal cardiac conduction system and the dynamic interplay between the sympathetic and parasympathetic branches of the fetal autonomic nervous system. While maternal factors and placental function can indirectly influence fetal heart rate (e.g., severe uteroplacental insufficiency leading to fetal hypoxia and subsequent FHR changes), the placenta itself does not directly regulate the fetal heart rate.
D. Surfactant is a complex lipoprotein mixture synthesized by type II alveolar cells within the fetal lungs, typically beginning around 20 weeks of gestation and increasing significantly between 32 and 36 weeks. Its primary role is to reduce alveolar surface tension, preventing atelectasis and ensuring proper lung expansion at birth. The placenta does not play a role in the synthesis of fetal surfactant.
Take home points
- The placenta is the primary organ for gas and nutrient exchange in utero.
- Fetal hemoglobin is produced by the fetus, not the placenta.
- Fetal heart rate is regulated by the fetal autonomic nervous system.
- Surfactant is synthesized in the fetal lungs for respiratory function.
Which vessel carries oxygenated blood from the placenta to the fetus?
Explanation
In fetal circulation, oxygenation of blood occurs not in the fetal lungs, but within the placenta, where maternal-fetal gas exchange takes place. The highly oxygenated blood is then transported to the fetus through a specialized vessel, ensuring that vital organs, particularly the developing brain and heart, receive a preferential supply of oxygen and nutrients. This system is designed to bypass the non-functional pulmonary circulation in utero.
Rationale for correct answers
C. The umbilical vein is the sole vessel within the umbilical cord responsible for transporting oxygenated and nutrient-rich blood from the placenta directly to the fetus. This vessel carries blood with an oxygen saturation of approximately 80-85%, which is the highest oxygen concentration in the entire fetal circulatory system.
Rationale for incorrect answers
A. The umbilical arteries are two vessels present in the umbilical cord that carry deoxygenated blood and metabolic waste products from the fetus back to the placenta. This deoxygenated blood has a lower oxygen saturation compared to the umbilical vein, as it has already circulated through the fetal tissues.
B. The ductus arteriosus is a critical fetal shunt that connects the pulmonary artery to the aorta, effectively bypassing the fetal lungs. In utero, due to high pulmonary vascular resistance, the majority of blood ejected from the right ventricle is shunted through the ductus arteriosus into the descending aorta, thereby diverting it away from the non-functional lungs.
D. The pulmonary artery in the fetus carries deoxygenated blood from the right ventricle towards the lungs. However, due to the high pulmonary vascular resistance and the presence of the ductus arteriosus, only a minimal amount of blood flows into the pulmonary circulation for the nutritional needs of the developing lung tissue.
Take home points
- The umbilical vein carries oxygenated blood from the placenta.
- The umbilical arteries carry deoxygenated blood from the fetus to the placenta.
- The ductus arteriosus is a fetal shunt bypassing the lungs.
- The pulmonary artery carries deoxygenated blood towards the fetal lungs.
A nurse is educating a patient about fetal circulation. Which structure allows blood to bypass the fetal liver?
Explanation
Fetal circulation uniquely incorporates several anatomical shunts that facilitate the bypass of developing but non-functional organs, such as the lungs and liver, in utero. These shunts are crucial for directing oxygenated blood preferentially to vital fetal organs like the brain and heart. The precise routing of blood through these temporary structures ensures optimal oxygen and nutrient delivery, circumventing high-resistance vascular beds.
Rationale for correct answers
B. The ductus venosus is the specific fetal shunt that allows a significant portion of the highly oxygenated blood from the umbilical vein to bypass the hepatic sinusoidal circulation and flow directly into the inferior vena cava. This strategic bypass ensures that well-oxygenated blood reaches the fetal heart with minimal impedance from the developing liver.
Rationale for incorrect answers
A. The foramen ovale is a fetal shunt located within the interatrial septum, connecting the right atrium directly to the left atrium. Its primary function is to shunt oxygenated blood, primarily from the inferior vena cava, away from the high-resistance pulmonary circulation and into the systemic circulation, thereby bypassing the fetal lungs. It does not bypass the fetal liver.
C. The ductus arteriosus is a fetal shunt that connects the pulmonary artery to the aorta. Its main purpose is to divert the majority of blood ejected from the right ventricle away from the fluid-filled, high-resistance pulmonary vasculature and into the systemic circulation, specifically the descending aorta. This shunt primarily bypasses the fetal lungs, not the fetal liver.
D. The pulmonary vein is a vessel in both fetal and adult circulation that carries oxygenated blood from the lungs to the left atrium. In the fetus, due to the minimal blood flow through the high-resistance pulmonary circulation, the amount of blood carried by the pulmonary vein is comparatively small. This vessel is an integral part of the pulmonary circulation and does not serve as a bypass for the fetal liver.
Take home points
- The ductus venosus bypasses the fetal liver.
- The foramen ovale bypasses the fetal lungs by shunting blood between atria.
- The ductus arteriosus bypasses the fetal lungs by shunting blood from the pulmonary artery to the aorta.
- Fetal shunts are crucial for optimizing blood flow to vital organs in utero.
What is the primary site of oxygenation for the fetus in utero?
Explanation
In the intrauterine environment, the developing fetus relies on a specialized organ for all its respiratory and metabolic exchanges. Unlike postnatal life where lungs assume the role of gas exchange, the fetal lungs are fluid-filled and have high vascular resistance, rendering them non-functional for oxygenation. Therefore, an alternative, highly efficient system is indispensable to ensure continuous oxygen supply and carbon dioxide removal.
Rationale for correct answers
C. The placenta serves as the primary and sole site of oxygenation for the fetus in utero. This highly vascularized organ facilitates the diffusion of oxygen from the maternal blood across the placental membrane into the fetal circulation, simultaneously removing carbon dioxide from the fetal blood for maternal excretion.
Rationale for incorrect answers
A. The fetal lungs, while developing, are filled with amniotic fluid and exhibit high pulmonary vascular resistance, making them inefficient for gas exchange in utero. Only a small fraction of fetal cardiac output, approximately 10-15%, perfuses the lungs for their growth and development, with the majority of blood bypassing them via fetal shunts.
B. The fetal liver is primarily involved in metabolic functions, hematopoiesis during early gestation, and storage of glycogen and iron. Although it receives some oxygenated blood from the umbilical vein, it is not the site of oxygenation for the fetal circulatory system as a whole. A significant portion of umbilical venous blood bypasses the liver via the ductus venosus.
D. The fetal heart is responsible for pumping blood throughout the fetal body, facilitating circulation and distribution of oxygen and nutrients to various tissues and organs. However, the heart itself does not perform gas exchange or oxygenation; rather, it receives oxygenated blood from the placenta via the umbilical vein.
Take home points
- The placenta is the fetal lung in utero.
- Fetal lungs are fluid-filled and non-functional for gas exchange.
- Fetal liver is for metabolism and hematopoiesis, not oxygenation.
- The fetal heart pumps oxygenated blood received from the placenta.
Which of the following are components of the fetal circulatory system? Select all that apply.
Explanation
The fetal circulatory system exhibits unique anatomical structures distinct from postnatal circulation, reflecting its adaptation to intrauterine oxygenation via the placenta. Key components include specialized vascular shunts that divert blood flow away from the non-functional lungs and partially functional liver. These adaptations ensure that the developing brain and heart receive preferential oxygen delivery.
Rationale for correct answers
A. The umbilical arteries are an essential component of the fetal circulatory system, responsible for carrying deoxygenated blood and metabolic waste products from the fetus back to the placenta for exchange with the maternal circulation. There are typically two umbilical arteries, which originate from the internal iliac arteries of the fetus.
C. The foramen ovale is a crucial fetal shunt located in the interatrial septum, allowing oxygenated blood to bypass the pulmonary circulation by flowing directly from the right atrium to the left atrium. This ensures that the most oxygenated blood is directed to the left side of the heart to supply the fetal brain and coronary arteries.
D. The ductus arteriosus is another vital fetal shunt, connecting the pulmonary artery directly to the aorta. This structure shunts the majority of blood away from the high-resistance pulmonary circulation and into the systemic circulation, thereby bypassing the non-functional fetal lungs in utero.
E. The superior vena cava is a major vein in the fetal circulatory system, as it is in adults, responsible for returning deoxygenated blood from the upper body, head, and upper extremities to the right atrium of the fetal heart. This blood then mixes with blood from the inferior vena cava.
Rationale for incorrect answers
B. The hepatic portal vein is a component of the adult circulatory system that primarily carries nutrient-rich, deoxygenated blood from the gastrointestinal tract and spleen to the liver for processing. While the fetal liver is perfused by branches of the umbilical vein and hepatic arteries, the typical adult-pattern hepatic portal vein system as the primary blood supply to the liver parenchyma is not fully established or as functionally significant in fetal circulation due to the dominant role of the umbilical vein and the presence of the ductus venosus. In fetal circulation, a significant portion of the oxygenated umbilical venous blood bypasses the liver via the ductus venosus, meaning the hepatic portal vein's role in fetal oxygenation is minimal and it does not function as a primary bypass or circulatory shunt.
Take home points
- Umbilical arteries, foramen ovale, ductus arteriosus, and superior vena cava are fetal circulatory components.
- Hepatic portal vein's role is minimal in fetal circulation due to ductus venosus.
- Fetal shunts are vital for bypassing non-functional organs in utero.
- Fetal circulation ensures preferential oxygen delivery to the brain and heart.
Practice Exercise 2
What is the normal range for baseline fetal heart rate (FHR)?
Explanation
The baseline fetal heart rate (FHR) represents the average FHR during a 10-minute segment, excluding periodic changes, periods of marked variability, and uterine contractions. It is a critical indicator of fetal oxygenation status and the overall well-being of the fetus. A stable baseline FHR reflects a balanced interplay between the sympathetic and parasympathetic divisions of the fetal autonomic nervous system.
Rationale for correct answers
C. The normal range for baseline fetal heart rate (FHR) in a term fetus is established as 110 to 160 beats per minute (bpm). This range signifies adequate oxygenation and a healthy, compensated fetal state, reflecting the normal physiological demands and autonomic nervous system regulation in utero.
Rationale for incorrect answers
A. A baseline fetal heart rate range of 80–100 bpm is considered fetal bradycardia. Fetal bradycardia is defined as a baseline FHR persistently below 110 bpm for 10 minutes or longer and can indicate significant fetal distress, such as severe hypoxia, congenital heart block, or profound maternal hypotension, necessitating urgent evaluation and intervention.
B. A baseline fetal heart rate range of 100–120 bpm, while closer to normal, still falls within the definition of fetal bradycardia if sustained for 10 minutes or more. Although less severe than rates below 100 bpm, it warrants careful assessment to rule out underlying causes of fetal compromise, as it deviates from the established normal range.
D. A baseline fetal heart rate range of 160–180 bpm is indicative of fetal tachycardia, which is defined as a baseline FHR persistently above 160 bpm for 10 minutes or longer. Fetal tachycardia can be a sign of various issues including maternal fever, maternal dehydration, chorioamnionitis, fetal hypoxia, or maternal use of certain medications, and requires clinical investigation.
Take home points
- Normal baseline FHR for a term fetus is 110-160 bpm.
- FHR <110 bpm is bradycardia, potentially indicating distress.
- FHR >160 bpm is tachycardia, warranting investigation for underlying causes.
- Baseline FHR reflects fetal oxygenation and autonomic nervous system function.
Which of the following is a cause of fetal tachycardia? Select all that apply.
Explanation
Fetal tachycardia is defined as a baseline fetal heart rate (FHR) persistently greater than 160 beats per minute (bpm) for a duration of 10 minutes or longer. It represents an increase in the fetal heart rate above the normal physiological range (110-160 bpm). This condition can be a significant indicator of various physiological stressors impacting the fetus, often reflecting an attempt by the fetal sympathetic nervous system to compensate for a compromised state.
Rationale for correct answers
B. Fetal hypoxia is a common and concerning cause of fetal tachycardia. In response to reduced oxygen availability, the fetal sympathetic nervous system is activated, leading to an increase in catecholamine release. This compensatory mechanism attempts to increase cardiac output and improve tissue perfusion in vital organs, resulting in an elevated fetal heart rate.
E. Maternal administration of beta-agonist medications, such as terbutaline, which are commonly used as tocolytics to suppress uterine contractions in preterm labor, can directly cause fetal tachycardia. These drugs stimulate beta-adrenergic receptors in both maternal and fetal cardiovascular systems, leading to an increase in heart rate.
Rationale for incorrect answers
A. Maternal hypotension typically leads to a decrease in uteroplacental perfusion, which can result in fetal bradycardia or late decelerations, rather than tachycardia. The reduced blood flow to the placenta diminishes oxygen delivery to the fetus, triggering a compensatory mechanism that often involves slowing of the heart rate in an attempt to conserve oxygen.
C. Umbilical cord compression typically manifests as variable decelerations in the fetal heart rate tracing. This is due to a transient reduction in blood flow through the umbilical vessels, leading to a sudden decrease in venous return and/or an increase in arterial resistance, which triggers a vagal response resulting in abrupt FHR drops, not sustained tachycardia.
D. Fetal head compression, often observed during uterine contractions in active labor, typically results in early decelerations of the fetal heart rate. This phenomenon occurs due to vagal nerve stimulation as the fetal head encounters resistance, causing a transient, symmetrical slowing of the heart rate that mirrors the uterine contraction. It does not cause fetal tachycardia.
Take home points
- Fetal tachycardia (>160 bpm for ≥10 min) can signify fetal hypoxia.
- Maternal beta-agonist medications can induce fetal tachycardia.
- Maternal hypotension usually causes bradycardia or late decelerations.
- Umbilical cord compression causes variable decelerations.
A nurse observes a fetal heart rate with absent variability. What does this finding suggest?
Explanation
Fetal heart rate (FHR) variability refers to the fluctuations in the baseline FHR that reflect the dynamic interplay between the sympathetic and parasympathetic branches of the fetal autonomic nervous system. It is considered the most reliable indicator of fetal oxygenation, acid-base status, and neurological integrity. Normal variability, known as moderate variability, is characterized by an amplitude range of 6-25 beats per minute (bpm).
Rationale for correct answers
B. Absent variability, defined as an amplitude range of the fetal heart rate fluctuations that is undetectable, is a highly concerning finding. It strongly suggests severe fetal hypoxia, metabolic acidemia, or significant neurological compromise, indicating an urgent need for intervention to prevent adverse fetal outcomes.
Rationale for incorrect answers
A. Normal fetal well-being is typically indicated by the presence of moderate variability, which is an amplitude range of 6-25 bpm. Absent variability, conversely, deviates significantly from this normal pattern and is a sign of potential fetal distress, not well-being.
C. Fetal movement often elicits accelerations in the fetal heart rate, which are transient increases in FHR above the baseline. While fetal movement can influence FHR, it is associated with normal variability or accelerations, not absent variability. Absent variability indicates a lack of autonomic nervous system response, which would contradict the presence of normal fetal movement.
D. Uterine contractions themselves are associated with changes in FHR patterns, such as early, late, or variable decelerations, depending on the physiological response. However, uterine contractions directly cause changes in the FHR pattern (periodic changes), and do not inherently cause or explain absent variability in the baseline FHR. Absent variability reflects an underlying state independent of the immediate mechanical effects of a contraction.
Take home points
- Absent FHR variability is a critical sign of severe fetal compromise.
- Moderate variability (6-25 bpm) indicates normal fetal well-being.
- Fetal movement typically causes FHR accelerations, not absent variability.
- Uterine contractions cause periodic FHR changes, not absent variability.
Which type of deceleration is associated with uteroplacental insufficiency?
Explanation
Fetal heart rate (FHR) decelerations are transient decreases in the FHR below the established baseline (normal range 110-160 bpm). These patterns provide critical insights into the fetal physiological response to various stressors, particularly during labor. Decelerations are categorized based on their morphology, timing relative to contractions, and relationship to underlying physiological mechanisms, aiding in the assessment of fetal oxygenation and well-being.
Rationale for correct answers
B. Late decelerations are precisely associated with uteroplacental insufficiency, which signifies a reduction in blood flow and oxygen transfer from the placenta to the fetus. This pattern is characterized by a gradual decrease in FHR that begins after the peak of the uterine contraction and returns to baseline only after the contraction has ended, indicating a delayed fetal response to recurrent hypoxemia during contractions.
Rationale for incorrect answers
A. Early decelerations are typically associated with fetal head compression during uterine contractions. They are characterized by a gradual decrease in FHR that mirrors the contraction, with the nadir of the deceleration coinciding with the peak of the contraction. This is generally considered a benign finding due to vagal stimulation and does not indicate uteroplacental insufficiency.
C. Variable decelerations are characterized by an abrupt, often irregular, decrease in FHR of varying shape, duration, and magnitude. These are most commonly caused by umbilical cord compression, which leads to a transient decrease in umbilical blood flow. While they can be concerning if severe or prolonged, their primary etiology is mechanical compression of the cord, not uteroplacental insufficiency.
D. A prolonged deceleration is a marked decrease in FHR of at least 15 bpm below the baseline, lasting for 2 minutes or more but less than 10 minutes. These decelerations can be caused by a variety of acute events that severely compromise fetal oxygenation, such as sustained umbilical cord compression, maternal hypotension, uterine tachysystole, or placental abruption. While uteroplacental insufficiency can contribute to a compromised fetus being less resilient to such events, it is not the primary or sole cause typically associated with the pattern of a prolonged deceleration itself.
Take home points
- Late decelerations specifically indicate uteroplacental insufficiency.
- Early decelerations are benign and linked to fetal head compression.
- Variable decelerations are caused by umbilical cord compression.
- Prolonged decelerations are acute FHR drops from various severe causes.
Which of the following are nursing responsibilities during electronic fetal monitoring? Select all that apply.
Explanation
Electronic fetal monitoring (EFM) is a crucial clinical tool used to assess fetal well-being by continuously recording the fetal heart rate (FHR) and uterine contractions. It allows for the real-time detection of FHR patterns that may indicate fetal compromise or an adequate oxygen supply. EFM encompasses both external and internal monitoring methods, providing vital data for clinical decision-making during labor and in high-risk pregnancies.
Rationale for correct answers
A. Assessing baseline FHR is a primary nursing responsibility during electronic fetal monitoring. Nurses must accurately identify the baseline FHR, which is the approximate mean FHR observed during a 10-minute segment, excluding accelerations, decelerations, and marked variability. The normal range for baseline FHR in a term fetus is 110-160 bpm.
B. Interpreting variability is a critical nursing responsibility. Variability, the fluctuations in the FHR baseline, reflects the integrity of the fetal autonomic nervous system and indicates fetal oxygenation. Nurses must distinguish between absent, minimal, moderate (6-25 bpm, reassuring), and marked variability to assess fetal well-being.
D. Documenting findings comprehensively and accurately is an essential nursing responsibility. This includes recording the baseline FHR, the presence and characteristics of variability, accelerations, and decelerations, as well as uterine contraction patterns, maternal vital signs, and any interventions performed, to ensure a complete and legally sound medical record.
E. Performing fetal stimulation, such as vibroacoustic stimulation (VAS) or scalp stimulation, is a nursing responsibility aimed at eliciting FHR accelerations to assess fetal reactivity, particularly during a non-reactive Non-Stress Test (NST). This maneuver helps differentiate between a sleep cycle and potential fetal compromise.
Rationale for incorrect answers
C. Administering tocolytics, which are medications used to suppress uterine contractions (e.g., in preterm labor or to reduce uterine hyperstimulation), is primarily within the scope of practice for the healthcare provider (physician, nurse practitioner, or midwife) who prescribes the medication. While nurses administer the prescribed medication, the decision to initiate or modify tocolytic therapy falls under the provider's responsibility, informed by the nurse's assessment findings from EFM. The nurse's role is to ensure safe administration, monitor for effectiveness, and assess for maternal and fetal side effects.
Take home points
- Nurses assess baseline FHR and interpret FHR variability.
- Comprehensive documentation of EFM findings is essential.
- Performing fetal stimulation is a nursing intervention to assess fetal reactivity.
- Administering tocolytics is a collaborative responsibility, initiated by a provider's order.
Practice Exercise 3
What happens to the ductus arteriosus after birth?
Explanation
The ductus arteriosus is a critical fetal circulatory shunt that connects the pulmonary artery to the aorta, bypassing the high-resistance pulmonary circulation in utero. Its functional and anatomical closure after birth is a pivotal physiological event that redirects blood flow to the lungs, enabling independent pulmonary respiration and establishing the adult pattern of systemic and pulmonary circulation.
Rationale for correct answers
C. After birth, the ductus arteriosus undergoes a rapid functional closure, typically within 10-15 hours, primarily in response to the abrupt increase in systemic oxygen tension and the decrease in circulating prostaglandin E2 (PGE2) levels upon removal of the placenta. Over the subsequent 2-3 weeks, it undergoes anatomical closure through fibrosis to become a non-functional fibrous remnant known as the ligamentum arteriosum.
Rationale for incorrect answers
A. The umbilical vein, not the ductus arteriosus, becomes the ligamentum teres (also known as the round ligament of the liver) after birth. The umbilical vein is responsible for carrying oxygenated blood from the placenta to the fetus, and its obliteration occurs after the cessation of placental blood flow.
B. The foramen ovale, a fetal shunt located in the interatrial septum, forms the fossa ovalis after birth. The functional closure of the foramen ovale occurs almost immediately due to the increased left atrial pressure and decreased right atrial pressure, with anatomical closure typically completed over several weeks to months.
D. If the ductus arteriosus remains patent after birth, it results in a condition called patent ductus arteriosus (PDA), which is a congenital heart defect, not a normal physiological outcome. A patent ductus arteriosus leads to left-to-right shunting of blood, causing pulmonary overcirculation and potentially heart failure, rather than supporting normal pulmonary circulation. Its closure is essential for establishing independent pulmonary circulation.
Take home points
- The ductus arteriosus closes to become the ligamentum arteriosum.
- Umbilical vein becomes the ligamentum teres.
- Foramen ovale becomes the fossa ovalis.
- Persistent patency of the ductus arteriosus (PDA) is a defect, not a normal state.
What physiological change immediately follows the clamping of the umbilical cord at birth?
Explanation
The transition from fetal to neonatal circulation involves a series of dramatic physiological changes that occur immediately after birth, enabling the newborn to adapt to extrauterine life. These changes are primarily driven by the cessation of placental blood flow and the onset of pulmonary respiration, leading to profound alterations in systemic and pulmonary vascular resistances, and the closure of fetal shunts.
Rationale for correct answers
C. An immediate increase in systemic vascular resistance (SVR) is a direct physiological consequence of clamping the umbilical cord. The placenta, being a low-resistance vascular bed, is removed from the circulatory system. This abrupt removal of the low-resistance placental circuit leads to a significant increase in the total peripheral resistance, causing a rapid rise in systemic blood pressure.
Rationale for incorrect answers
A. A significant decrease in systemic vascular resistance is incorrect. As explained, the removal of the low-resistance placental circuit leads to an increase in systemic vascular resistance, not a decrease. This increase is crucial for elevating systemic blood pressure and facilitating the postnatal circulatory changes.
B. A dramatic increase in pulmonary vascular resistance (PVR) is incorrect. In fetal life, PVR is high due to the unexpanded, fluid-filled lungs and hypoxic pulmonary vasoconstriction. At birth, with the first breaths and the expansion of the lungs, oxygen tension in the alveoli increases dramatically. This rise in oxygen tension, along with mechanical expansion, causes a rapid and dramatic decrease in pulmonary vascular resistance, allowing for a significant increase in pulmonary blood flow.
D. A decrease in aortic pressure is incorrect. Following the clamping of the umbilical cord and the resultant increase in systemic vascular resistance, there is an immediate and substantial increase in aortic pressure. This rise in systemic pressure is vital for reversing the pressure gradients across fetal shunts (like the foramen ovale and ductus arteriosus) and establishing the adult pattern of circulation.
Take home points
- Clamping the umbilical cord immediately increases systemic vascular resistance.
- The placenta's removal eliminates a low-resistance vascular bed.
- Pulmonary vascular resistance decreases significantly with lung expansion and oxygenation.
- Aortic pressure increases after cord clamping due to increased SVR.
Which of the following are changes that occur in the fetal shunts at birth due to increased oxygen levels and pressure changes? Select all that apply.
Explanation
Fetal circulation transition refers to the physiologic changes that occur after birth as the newborn shifts from placental oxygenation to pulmonary gas exchange. Three fetal shunts—ductus arteriosus, ductus venosus, and foramen ovale—bypass the lungs and liver in utero. At birth, increased systemic vascular resistance, decreased pulmonary vascular resistance, and rising oxygen tension lead to functional closure of these shunts. Normal neonatal oxygen saturation increases to >95%, triggering prostaglandin inhibition and muscular constriction of the shunts. Pulmonary flow increases dramatically as alveoli expand, enabling efficient oxygen uptake.
Rationale for correct answers
A. Functional closure of the foramen ovale occurs within the first few minutes after birth due to increased left atrial pressure exceeding right atrial pressure. This pressure change prevents right-to-left shunting.
B. Functional closure of the ductus arteriosus occurs due to elevated oxygen levels and reduced prostaglandins, leading to constriction of the ductal smooth muscle. Complete anatomic closure follows within 1–2 weeks.
D. Increased flow through the pulmonary artery happens as lung expansion reduces pulmonary vascular resistance, enabling the right ventricle to direct blood into the pulmonary circulation for gas exchange.
E. Reversal of blood flow in the umbilical arteries occurs after clamping of the cord. Systemic pressure rises, and the formerly low-resistance placental circuit is eliminated, halting placental blood flow.
Rationale for incorrect answers
C. The ductus venosus does not open at birth; rather, it functionally closes as venous return from the umbilical vein ceases. Its closure is triggered by the cessation of placental blood flow following cord clamping, not by opening. Over days, it fibroses into the ligamentum venosum.
Take home points
- Fetal shunt closures are triggered by increased oxygen levels and pressure shifts post-birth.
- Foramen ovale closes due to higher left atrial pressure.
- Ductus arteriosus closes due to oxygen-induced vasoconstriction.
- Umbilical blood flow ceases after cord clamping, stopping placental circulation.
What causes the dramatic decrease in pulmonary vascular resistance (PVR) at birth?
Explanation
Pulmonary vascular resistance (PVR) at birth decreases due to mechanical, chemical, and oxygenation changes that enable pulmonary circulation to replace placental gas exchange. In utero, PVR is high due to collapsed alveoli and low oxygen tension. After birth, lung expansion, oxygen increase, alveolar vasodilation, and pulmonary endothelial nitric oxide release drastically reduce PVR. Normal neonatal PaO₂ increases from <25 mmHg in utero to >60 mmHg postnatally. This enables efficient gas exchange, left-to-right shunting in transitional fetal structures, and redirection of cardiac output through pulmonary arteries.
Rationale for correct answers
C. Lung expansion and fluid clearance increase alveolar oxygen tension, leading to relaxation of pulmonary arterioles. This mechanical distension plus increased oxygen lowers pulmonary vascular tone, causing the dramatic fall in PVR required for postnatal gas exchange.
Rationale for incorrect answers
A. Prostaglandin E2 maintains fetal circulation by keeping the ductus arteriosus patent. Increased levels do not reduce PVR; in fact, decreased prostaglandin levels after birth contribute to ductal closure. High prostaglandin E2 sustains high PVR in utero by promoting vasoconstriction in the pulmonary bed.
B. Clamping of the umbilical cord increases systemic vascular resistance, not pulmonary vascular resistance. While it redirects blood flow away from the placenta, it does not directly cause the drop in PVR. The decrease in PVR depends on alveolar oxygenation and mechanical expansion, not cord clamping.
D. Closure of the ductus arteriosus is a consequence of increased oxygen and decreased prostaglandins after PVR drops. It follows PVR changes rather than causes them. Its closure helps establish adult circulation patterns but is not the initiating event for PVR reduction.
Take home points
- PVR falls due to lung expansion and increased oxygen after birth.
- Prostaglandin levels must decrease for fetal shunt closure.
- Umbilical cord clamping increases systemic, not pulmonary, resistance.
- Ductus arteriosus closes after PVR has already begun to fall.
Select all that apply.
Explanation
Fetal circulation oxygenation is characterized by preferential shunting and oxygen gradient preservation to ensure that the most oxygenated blood reaches vital structures like the heart and brain. Oxygenated blood from the placenta enters the fetus via the umbilical vein with an oxygen saturation of approximately 80%, the highest in fetal circulation. Blood is mixed at several levels due to shunting via the ductus venosus, foramen ovale, and ductus arteriosus, resulting in varying oxygen saturation levels throughout the fetal body. Average fetal systemic oxygen saturation is around 60%–70%, which is lower than the normal adult arterial range of 95%–100%. Hypoxic vasoconstriction in the fetal lungs maintains high pulmonary vascular resistance and promotes systemic shunting.
Rationale for correct answers
A. The umbilical vein carries oxygen-rich blood from the placenta with an oxygen saturation of approximately 80%. It is the most oxygenated vessel in fetal circulation, delivering blood to the ductus venosus and then into the inferior vena cava.
C. Blood entering the left ventricle via the foramen ovale is relatively well oxygenated (65%–70%) due to the preferential streaming of the more oxygenated inferior vena cava blood across the atrial septum. This blood is directed to the coronary and cerebral circulations.
E. Fetal oxygen saturation levels are significantly lower than in adults. Mixed blood in the aorta and systemic circulation has oxygen saturations ranging between 50%–70%, reflecting the inefficiency of placental gas exchange compared to postnatal pulmonary oxygenation.
Rationale for incorrect answers
B. Blood in the descending aorta is less oxygenated than in the ascending aorta. This is due to the admixture of deoxygenated blood from the ductus arteriosus entering the descending aorta, which reduces its oxygen content compared to the ascending aorta that receives better-oxygenated blood from the left ventricle.
D. The pulmonary artery in fetal life carries partially oxygenated blood, not fully deoxygenated. The blood is a mixture from the right ventricle (mostly deoxygenated) and systemic venous return, with an oxygen saturation around 55%–60%. Total deoxygenation does not occur in fetal vessels due to continuous mixing.
Take home points
- Umbilical vein carries the most oxygenated blood in the fetus.
- Blood reaching the left ventricle is prioritized for the brain and heart.
- Fetal oxygen saturation is significantly lower than postnatal levels.
- Mixing of blood at fetal shunts results in variable oxygenation across vessels.
Comprehensive Questions
What is the primary purpose of the foramen ovale in fetal circulation?
Explanation
Foramen ovale is a key fetal circulatory shunt that enables oxygenated blood from the inferior vena cava (IVC) to bypass the nonfunctional fetal lungs by flowing directly from the right atrium to the left atrium. This facilitates preferential delivery of relatively well-oxygenated blood to the coronary arteries and brain via the left ventricle and ascending aorta. In fetal life, the lungs are collapsed, and pulmonary vascular resistance is high, so blood must be redirected through anatomical shunts. The normal fetal heart rate ranges from 110 to 160 bpm, and systemic oxygen saturation averages 60%–70%, much lower than postnatal levels.
Rationale for correct answers
B. The foramen ovale allows blood to flow directly from the right atrium to the left atrium, bypassing the high-resistance pulmonary circulation. This ensures that relatively oxygenated blood from the IVC is routed to the left ventricle and systemic circulation to supply vital organs.
Rationale for incorrect answers
A. Blood is not shunted from the right ventricle to the aorta through the foramen ovale. That function is carried out by the ductus arteriosus, which connects the pulmonary artery to the descending aorta, not by the foramen ovale.
C. The ductus venosus, not the foramen ovale, bypasses the liver by channeling oxygenated blood from the umbilical vein directly to the IVC. The foramen ovale plays no role in hepatic circulation.
D. The umbilical arteries return deoxygenated blood to the placenta, not the foramen ovale. These arteries arise from the internal iliac arteries and transport waste-laden blood to the placenta for oxygen exchange.
Take home points
- The foramen ovale shunts blood from the right atrium to the left atrium.
- It bypasses the nonfunctioning fetal lungs.
- Ductus arteriosus connects the pulmonary artery to the aorta.
- Ductus venosus bypasses the liver by shunting blood into the IVC.
Which blood vessel carries oxygenated and nutrient-rich blood from the placenta to the fetus?
Explanation
Umbilical circulation in fetal life is specialized to facilitate oxygen and nutrient exchange between the fetus and the placenta. The umbilical vein is the only fetal vessel that carries oxygenated and nutrient-rich blood from the placenta directly toward the fetus. Blood in this vessel has an oxygen saturation of approximately 80%, the highest in fetal circulation. After entering the fetus at the umbilicus, blood from the umbilical vein bypasses the hepatic microcirculation through the ductus venosus and enters the inferior vena cava (IVC), where it mixes with deoxygenated blood before reaching the heart.
Rationale for correct answers
C. The umbilical vein carries highly oxygenated, nutrient-rich blood from the placenta to the fetus. It travels from the placenta through the umbilical cord and connects to the ductus venosus, delivering blood to the IVC and then to the right atrium.
Rationale for incorrect answers
A. The umbilical arteries carry deoxygenated, nutrient-poor blood from the fetus back to the placenta. They originate from the internal iliac arteries and are responsible for eliminating waste and carbon dioxide via placental exchange.
B. The pulmonary artery carries blood from the right ventricle to the fetal lungs. However, in fetal life, the lungs are nonfunctional, and most of this blood is diverted through the ductus arteriosus to the descending aorta. This blood is deoxygenated.
D. The aorta distributes mixed oxygenated blood to the fetal body. Although it carries blood to systemic tissues, it does not deliver blood from the placenta and is not the most oxygenated vessel.
Take home points
- The umbilical vein carries oxygenated blood from the placenta to the fetus.
- It has the highest oxygen saturation in fetal circulation (~80%).
- The umbilical arteries return deoxygenated blood to the placenta.
- The ductus venosus allows umbilical vein blood to bypass the liver.
Which structure connects the pulmonary artery to the aorta in the fetus?
Explanation
Ductus arteriosus is a vital fetal vascular connection between the pulmonary artery and the descending aorta, allowing most of the right ventricular output to bypass the nonfunctioning fetal lungs. In utero, pulmonary vascular resistance is high due to collapsed alveoli and low oxygen tension, diverting blood through the ductus arteriosus to the aorta. This shunt ensures systemic perfusion, particularly to the lower body and placenta. Closure of the ductus arteriosus occurs functionally within 10–15 hours after birth due to increased oxygen tension and decreased prostaglandin E2, with full anatomical closure by 2–3 weeks of life.
Rationale for correct answers
C. The ductus arteriosus connects the pulmonary artery to the descending aorta. It allows most of the blood from the right ventricle to bypass the high-resistance pulmonary circulation and enter the systemic circulation directly, critical for fetal oxygen distribution.
Rationale for incorrect answers
A. The ductus venosus connects the umbilical vein to the inferior vena cava, bypassing the hepatic circulation. It plays no role in pulmonary or aortic circulation.
B. The foramen ovale is an interatrial opening that allows blood to shunt from the right atrium to the left atrium, bypassing the lungs. It does not connect the pulmonary artery to the aorta.
D. The umbilical vein carries oxygenated blood from the placenta to the fetus. It enters the ductus venosus and does not connect to either the pulmonary artery or aorta.
Take home points
- Ductus arteriosus connects pulmonary artery to aorta in fetal circulation.
- It allows right ventricular output to bypass the lungs.
- Functional closure is triggered by increased oxygen and decreased prostaglandins.
- Failure to close postnatally results in patent ductus arteriosus.
What is the name of the fetal shunt that bypasses the fetal liver?
Explanation
Ductus venosus is the fetal vascular shunt that bypasses the liver, allowing oxygenated blood from the umbilical vein to flow directly into the inferior vena cava (IVC). This adaptation ensures that the most highly oxygenated blood (with an oxygen saturation around 80%) reaches the heart and brain quickly without undergoing hepatic filtration. In fetal circulation, the liver receives only a small portion of the oxygenated umbilical blood, as the ductus venosus diverts the majority. After birth, this shunt closes functionally within minutes to hours, with complete anatomical closure within 1–2 weeks, becoming the ligamentum venosum.
Rationale for correct answers
C. The ductus venosus is the fetal shunt that connects the umbilical vein to the IVC, bypassing the liver. It allows the majority of oxygen-rich placental blood to enter the systemic circulation directly, prioritizing perfusion to critical fetal organs.
Rationale for incorrect answers
A. The foramen ovale is an atrial-level shunt that bypasses the fetal lungs by directing blood from the right atrium to the left atrium. It plays no role in hepatic circulation.
B. The ductus arteriosus connects the pulmonary artery to the descending aorta, bypassing the lungs. It does not affect liver perfusion.
D. The ligamentum teres is the fibrous remnant of the umbilical vein after birth. It does not function as a shunt and is not present during fetal circulation as a bypass mechanism.
Take home points
- The ductus venosus bypasses the fetal liver by shunting blood to the IVC.
- It allows rapid delivery of oxygenated blood from the placenta to the heart.
- Postnatal closure forms the ligamentum venosum.
- Other fetal shunts include foramen ovale (atria) and ductus arteriosus (pulmonary artery to aorta).
Which of the following triggers the closure of the foramen ovale at birth?
Explanation
Closure of the foramen ovale at birth is a direct result of hemodynamic changes associated with the transition from placental to pulmonary gas exchange. As the newborn breathes, the pulmonary vascular resistance drops, allowing increased pulmonary blood flow and greater return to the left atrium. Simultaneously, clamping the umbilical cord increases systemic vascular resistance, raising left-sided pressures. The foramen ovale, a flap-like opening between the atria, functionally closes when left atrial pressure exceeds right atrial pressure, preventing right-to-left shunting. Functional closure typically occurs within minutes after birth; anatomical closure completes by 6 months.
Rationale for correct answers
C. Increased left atrial pressure relative to the right atrium forces the septum primum against the septum secundum, functionally closing the foramen ovale. This pressure shift is caused by enhanced pulmonary venous return following lung expansion.
Rationale for incorrect answers
A. Pulmonary vascular resistance decreases, not increases, after birth due to lung aeration and increased alveolar oxygen. A decrease in PVR facilitates increased left atrial return, contributing to closure—not an increase.
B. Oxygen levels rise after birth, not decrease. Increased oxygen tension is essential for pulmonary vasodilation and shunt closure. Decreased oxygen would maintain fetal circulatory pathways, not close them.
D. Clamping of the umbilical arteries contributes to systemic vascular resistance but does not directly trigger foramen ovale closure. It affects ductus venosus and arterial pressures, not atrial-level dynamics directly.
Take home points
- Foramen ovale closes due to increased left atrial pressure after birth.
- Pulmonary blood flow increases after PVR drops with lung expansion.
- Functional closure happens within minutes; anatomic closure within months.
- Right-to-left shunting stops when the atrial pressure gradient reverses.
What is the definition of a fetal heart rate acceleration in a term fetus?
Explanation
Fetal heart rate monitoring in term fetuses involves analyzing baseline patterns for signs of well-being during labor. Accelerations, baseline variability, gestational age, and oxygenation status guide interpretation. In a healthy term fetus, normal baseline fetal heart rate ranges from 110–160 bpm. Accelerations reflect transient increases in sympathetic tone, often linked to fetal movement or stimulation, and suggest adequate oxygenation and autonomic responsiveness.
Rationale for correct answers
B. An acceleration is defined as an increase in fetal heart rate of ≥15 bpm lasting ≥15 seconds in fetuses ≥32 weeks gestation. This pattern correlates with intact neurological regulation and absence of fetal hypoxia.
Rationale for incorrect answers
A. Increase of ≥10 bpm for ≥10 seconds defines an acceleration only in fetuses <32 weeks. In a term fetus, this threshold is too low and does not qualify as a valid acceleration under standard criteria.
C. A decrease in fetal heart rate of ≥15 bpm for ≥15 seconds defines a deceleration, not an acceleration. It may indicate cord compression, placental insufficiency, or uterine hypertonicity depending on its timing and morphology.
D. A decrease of ≥10 bpm for ≥10 seconds also constitutes a mild deceleration pattern and cannot be interpreted as an acceleration. Such decreases do not reflect fetal well-being but may warrant clinical observation.
Take home points
● A fetal heart rate acceleration in term fetuses is ≥15 bpm lasting ≥15 seconds.
● Accelerations signal intact autonomic and oxygenation status.
● Decelerations are characterized by decreases in bpm and may reflect pathology.
● Gestational age determines interpretation thresholds for accelerations.
What is the primary role of the umbilical arteries in fetal circulation?
Explanation
Fetal circulatory architecture establishes a placental-dependent system where oxygen delivery, waste removal, pressure gradients, and vascular routing are coordinated through specialized shunts. The umbilical arteries originate from the internal iliac arteries and carry blood with oxygen saturation approximately 50–60%. Fetal arterial PaO₂ is around 20–25 mmHg, significantly lower than postnatal arterial values of 80–100 mmHg. Low oxygen content is tolerated due to high fetal hemoglobin concentration.
Rationale for correct answers
B. The umbilical arteries are responsible for transporting deoxygenated, waste-laden blood from the fetus to the placenta. Their origin from the internal iliac arteries and their direct routing to the placenta supports excretory and respiratory exchange with maternal circulation.
Rationale for incorrect answers
A. The umbilical arteries do not deliver oxygenated blood. Instead, oxygenated blood is supplied to the fetus by the umbilical vein, which carries blood from the placenta with saturation levels around 70–80%. This blood bypasses hepatic circulation via the ductus venosus en route to systemic circulation.
C. The fetal lungs are bypassed by other shunting mechanisms—not the umbilical arteries. Structures like the foramen ovale and ductus arteriosus direct blood away from nonfunctional fetal lungs. The umbilical arteries are peripheral and not directly involved in this bypass system.
D. Nutrient absorption occurs via the placenta and maternal supply, not through the umbilical arteries. These arteries merely transport deoxygenated blood to the placenta, where nutrient and gas exchange occurs. They have no absorptive role.
Take home points
- Umbilical arteries carry deoxygenated blood from fetus to placenta.
- Oxygenated blood is supplied via the umbilical vein, not arteries.
- Fetal lungs are bypassed by cardiac shunts, not vascular exits.
- Nutrient absorption is a placental function, not arterial.
What does a reactive non-stress test (NST) indicate?
Explanation
Non-stress test interpretation evaluates fetal heart rate patterns in response to movement, primarily assessing oxygenation, autonomic integrity, placental function, and neurologic status. In term fetuses, normal baseline fetal heart rate ranges from 110–160 bpm with variability of 6–25 bpm. A reactive NST shows accelerations of ≥15 bpm lasting ≥15 seconds, suggesting intact sympathetic and parasympathetic regulation and adequate fetal oxygenation.
Rationale for correct answers
B. A reactive non-stress test reflects fetal well-being. It indicates sufficient oxygen delivery via the placenta and intact autonomic response. The presence of at least 2 accelerations within a 20-minute window in term fetuses confirms reactivity and a reassuring status.
Rationale for incorrect answers
A. Fetal distress is typically associated with absent accelerations, decreased variability, or late decelerations. A reactive NST, by definition, rules out distress at the time of testing and suggests normal fetal acid–base balance.
C. Uteroplacental insufficiency manifests as absent accelerations, decreased variability, or late decelerations. These would lead to a non-reactive NST, not a reactive one, which implies that placental perfusion is adequate.
D. Cord compression leads to variable decelerations, not accelerations. If present, these would be noted during monitoring and may signal concern depending on frequency and severity. A reactive NST lacks these deceleration patterns.
Take home points
- Reactive NST suggests adequate fetal oxygenation and neurologic status.
- Requires 2 accelerations of ≥15 bpm lasting ≥15 seconds in 20 minutes.
- Non-reactive NST may signal placental or umbilical pathology.
What is the primary reason fetal hemoglobin (HbF) has a higher affinity for oxygen than adult hemoglobin (HbA)?
Explanation
Oxygen affinity of fetal hemoglobin is regulated by molecular structure and interaction with intracellular modulators such as 2,3-bisphosphoglycerate (2,3-BPG). Differences in subunit composition, oxygen binding dynamics, hemoglobin dissociation curve, and blood saturation levels explain why fetal hemoglobin is better suited for placental gas exchange. HbF binds oxygen more tightly than HbA due to reduced sensitivity to 2,3-BPG; PaO₂ in umbilical venous blood is typically 30–35 mmHg with oxygen saturation around 70–80%.
Rationale for correct answers
B. Fetal hemoglobin (HbF) is composed of two alpha and two gamma chains (α₂γ₂), unlike adult hemoglobin (HbA) which has two alpha and two beta chains (α₂β₂). The γ chains have reduced affinity for 2,3-BPG, an allosteric modulator that normally decreases hemoglobin's oxygen affinity. Less binding of 2,3-BPG allows HbF to retain higher oxygen affinity, critical for extracting oxygen from maternal blood across the placenta.
Rationale for incorrect answers
A. Hemoglobin size does not influence oxygen affinity. Both HbF and HbA have tetrameric structures and comparable molecular weight (~64 kDa). Oxygen binding characteristics are not determined by molecular size but by subunit interactions and responsiveness to modulators like 2,3-BPG.
C. Carbon dioxide transport is mediated through different mechanisms including carbamino formation and bicarbonate buffering. HbF does not demonstrate superior carbon dioxide carriage over HbA. The increased oxygen affinity of HbF is unrelated to carbon dioxide handling.
D. While HbF concentration in fetal blood is higher than HbA concentration in adults (about 70–90% of total hemoglobin in the fetus), oxygen affinity is not determined by quantity. It is a function of molecular structure and reduced modulation by 2,3-BPG. Concentration influences oxygen-carrying capacity, not affinity.
Take home points
- HbF’s α₂γ₂ structure leads to lower 2,3-BPG binding and higher oxygen affinity.
- HbF is adapted for efficient oxygen uptake from low PaO₂ placental blood.
- Oxygen affinity is determined by molecular composition, not hemoglobin concentration.
- HbF's oxygen affinity facilitates fetal survival in relatively hypoxic intrauterine conditions.
What is the oxygen saturation of blood in the umbilical vein?
Explanation
Umbilical vein oxygen saturation reflects placental oxygen transfer efficiency and is central to fetal gas exchange. Oxygen tension, placental perfusion, fetal hemoglobin, and venous routing regulate oxygen delivery. PaO₂ in the umbilical vein ranges from 30–35 mmHg with oxygen saturation of approximately 70–80%. This relatively low oxygen tension is compensated by high fetal hemoglobin concentration and affinity.
Rationale for correct answers
C. The oxygen saturation of blood in the umbilical vein is about 70–80%. This vein carries oxygenated blood from the placenta to the fetus. Although PaO₂ remains low (30–35 mmHg), high levels of fetal hemoglobin (HbF) and its affinity ensure sufficient oxygen delivery.
Rationale for incorrect answers
A. An oxygen saturation of 20% is far below physiological levels for umbilical venous blood. Saturation this low would indicate profound hypoxia incompatible with fetal survival. Venous blood from the placenta is enriched and never falls to this range under normal conditions.
B. A saturation of 50% is typical of mixed blood seen in fetal systemic circulation or the pulmonary artery, not the umbilical vein. This value reflects oxygen levels after blood mixing with deoxygenated venous return, not direct placental transfer.
D. A saturation of 100% is unattainable in the umbilical vein due to low placental PaO₂ (~50 mmHg) and diffusion limitations. Maternal arterial oxygen saturation may be near 98–100%, but fetal venous saturation remains substantially lower due to fetal metabolic activity and limited oxygen gradient.
Take home points
- Umbilical vein oxygen saturation averages 70–80%.
- PaO₂ in umbilical venous blood is 30–35 mmHg.
- Fetal hemoglobin facilitates oxygen uptake at low PaO₂.
- Saturation values over 90% are never observed in fetal veins.
Which of the following are functions of the placenta in fetal circulation? Select all that apply.
Explanation
Placental function in fetal circulation ensures survival and growth in utero through tightly coordinated gas exchange, nutrient delivery, waste removal, and hormonal signaling. The placenta acts as a surrogate lung, kidney, and gastrointestinal interface. Oxygen transfer occurs at low PaO₂ levels (30–35 mmHg), yet fetal hemoglobin ensures adequate tissue oxygenation. It also facilitates diffusion of glucose, amino acids, and removal of metabolic by-products such as urea and CO₂.
Rationale for correct answers
A. The placenta facilitates gas exchange by transferring oxygen from maternal blood to fetal blood and removing carbon dioxide. This exchange occurs at the chorionic villi without mixing blood directly. Umbilical vein oxygen saturation typically reaches 70–80%.
B. Placental villi mediate active and passive transport of nutrients including glucose, amino acids, fatty acids, and micronutrients from maternal circulation to the fetus. Transport systems ensure nutrient delivery despite varying maternal serum concentrations.
D. Waste products like carbon dioxide, urea, and bilirubin are transferred from fetal to maternal blood via the placenta for excretion. The placenta essentially substitutes renal and hepatic clearance during fetal development.
Rationale for incorrect answers
C. Fetal hemoglobin (HbF) is synthesized within the fetal liver and bone marrow, not by the placenta. HbF appears from the 6th week of gestation and becomes predominant by the second trimester. The placenta plays no direct role in hemoglobin synthesis.
E. The placenta does not directly regulate fetal heart rate. Heart rate is modulated by fetal autonomic nervous system development, oxygenation status, and neurological integrity. While placental function influences oxygenation, it does not exert active cardiac regulation.
Take home points
- Placenta replaces fetal lungs and kidneys in oxygen and waste exchange.
- Delivers essential nutrients from maternal circulation to fetal tissues.
- HbF synthesis occurs in fetal liver, not placenta.
- Fetal heart rate is regulated neurologically, not placentally.
Which of the following are fetal circulatory shunts? Select all that apply.
Explanation
Fetal circulatory shunts are specialized structures that redirect blood flow to optimize oxygen delivery and bypass non-functional organs like the lungs and liver during fetal development. The ductus venosus, foramen ovale, and ductus arteriosus are critical for maintaining fetal circulation. These shunts close postnatally, transitioning to adult circulation. Normal fetal arterial oxygen saturation is 20–25%, and venous oxygen saturation is 70–80%.
Rationale for correct answers
A. The ductus venosus shunts oxygenated blood from the umbilical vein directly to the inferior vena cava, bypassing the liver. This ensures that highly oxygenated blood reaches the heart and brain efficiently.
B. The foramen ovale is an opening between the right and left atria that allows oxygenated blood from the inferior vena cava to bypass the non-functional fetal lungs and flow directly into systemic circulation.
D. The ductus arteriosus connects the pulmonary artery to the descending aorta, diverting blood away from the lungs and into systemic circulation. This shunt ensures that blood is distributed to the lower body and placenta for oxygenation.
Rationale for incorrect answers
C. The umbilical artery is not a shunt but a vessel that carries deoxygenated blood and waste products from the fetus to the placenta. It does not redirect blood flow within the fetal circulatory system.
E. The pulmonary vein carries oxygenated blood from the lungs to the left atrium in postnatal circulation. In fetal circulation, the lungs are non-functional, and the pulmonary vein does not play a significant role.
Take home points
- Fetal circulatory shunts include the ductus venosus, foramen ovale, and ductus arteriosus.
- These shunts bypass the liver and lungs to optimize oxygen delivery.
- The umbilical artery and pulmonary vein are not shunts but regular vessels.
Postnatal closure of shunts transitions circulation to the adult pattern
Which of the following factors contribute to the significant increase in systemic vascular resistance (SVR) at birth? Select all that apply.
Explanation
A. Clamping of the umbilical cord eliminates the low-resistance placental circulation, which previously accounted for a significant portion of fetal blood flow. This increases systemic vascular resistance as blood is now redirected through the neonatal systemic circulation.
B. The first breath and lung expansion lead to a dramatic decrease in pulmonary vascular resistance, which increases left atrial pressure. This shift in pressure gradients contributes to the closure of fetal shunts and an increase in systemic vascular resistance.
D. Closure of the foramen ovale occurs due to increased left atrial pressure following lung expansion and decreased right atrial pressure after umbilical cord clamping. This closure redirects blood flow through the systemic circulation, contributing to the rise in systemic vascular resistance.
Rationale for incorrect answers
C. Prostaglandin E2 levels decrease at birth, not increase. This decline facilitates the closure of the ductus arteriosus and other fetal shunts. Increased prostaglandin E2 would maintain patency of these shunts, opposing the rise in systemic vascular resistance.
E. Activation of the renal system is not an immediate factor in the increase in systemic vascular resistance at birth. While renal function begins to regulate fluid and electrolyte balance postnatally, it does not directly influence the acute changes in vascular resistance during the transition to neonatal circulation.
Take home points
- Clamping the umbilical cord eliminates the low-resistance placental circuit, increasing SVR.
- Lung expansion reduces pulmonary vascular resistance and redirects blood flow.
- Closure of fetal shunts, including the foramen ovale, contributes to increased SVR.
- Prostaglandin E2 levels decrease at birth, facilitating shunt closure.
Which of the following are components of a biophysical profile (BPP)? Select all that apply.
Explanation
Biophysical profile (BPP) is a prenatal test used to assess fetal well-being by evaluating fetal heart rate, breathing movements, tone, and amniotic fluid volume. It combines ultrasound and non-stress test findings. Normal BPP scores range from 8–10, indicating low risk of fetal compromise, while scores below 6 suggest potential hypoxia or acidosis.
Rationale for correct answers
A. Fetal heart rate accelerations are assessed using a non-stress test, which evaluates the fetal response to movement. Two or more accelerations of at least 15 beats per minute lasting 15 seconds within 20 minutes are considered normal.
B. Fetal breathing movements are observed via ultrasound. Normal breathing movements include rhythmic diaphragmatic contractions lasting at least 30 seconds within a 30-minute observation period.
D. Fetal tone is assessed by observing movements such as limb flexion and extension. Normal tone includes at least one episode of active extension with return to flexion within 30 minutes.
Rationale for incorrect answers
C. Maternal blood pressure is not a component of the biophysical profile. While maternal health is critical for fetal well-being, blood pressure is assessed separately and does not directly contribute to the BPP score.
E. Cervical length is not evaluated in a biophysical profile. It is assessed during pregnancy to predict preterm labor risk but is unrelated to fetal well-being parameters measured in the BPP.
Take home points
- Biophysical profile evaluates fetal heart rate, breathing movements, tone, and amniotic fluid volume.
- Normal BPP scores range from 8–10; scores below 6 indicate potential fetal compromise.
- Maternal blood pressure and cervical length are not components of the BPP.
- BPP combines ultrasound findings with non-stress test results.
Which of the following are components of a biophysical profile (BPP)? Select all that apply.
Explanation
Biophysical profile (BPP) is a prenatal test used to assess fetal well-being by evaluating fetal heart rate, breathing movements, tone, and amniotic fluid volume. It combines ultrasound and non-stress test findings. Normal BPP scores range from 8–10, indicating low risk of fetal compromise, while scores below 6 suggest potential hypoxia or acidosis.
Rationale for correct answers
A. Fetal heart rate accelerations are assessed using a non-stress test, which evaluates the fetal response to movement. Two or more accelerations of at least 15 beats per minute lasting 15 seconds within 20 minutes are considered normal.
B. Fetal breathing movements are observed via ultrasound. Normal breathing movements include rhythmic diaphragmatic contractions lasting at least 30 seconds within a 30-minute observation period.
D. Fetal tone is assessed by observing movements such as limb flexion and extension. Normal tone includes at least one episode of active extension with return to flexion within 30 minutes.
Rationale for incorrect answers
C. Maternal blood pressure is not a component of the biophysical profile. While maternal health is critical for fetal well-being, blood pressure is assessed separately and does not directly contribute to the BPP score.
E. Cervical length is not evaluated in a biophysical profile. It is assessed during pregnancy to predict preterm labor risk but is unrelated to fetal well-being parameters measured in the BPP.
Take home points
- Biophysical profile evaluates fetal heart rate, breathing movements, tone, and amniotic fluid volume.
- Normal BPP scores range from 8–10; scores below 6 indicate potential fetal compromise.
- Maternal blood pressure and cervical length are not components of the BPP.
- BPP combines ultrasound findings with non-stress test results.
Exams on Fetal Circulation
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
Notes Highlighting is available once you sign in. Login Here.
Objectives
➤ To elucidate the intricate anatomical structures and physiological mechanisms that characterize fetal circulation, distinguishing it from postnatal cardiovascular dynamics.
➤ To analyze the specialized fetal circulatory shunts—ductus venosus, foramen ovale, and ductus arteriosus—and their pivotal roles in bypassing non-functional organs, specifically the pulmonary and hepatic systems.
➤ To comprehend the unique properties of fetal hemoglobin (HbF) and its critical function in optimizing oxygen transfer from the maternal to the fetal circulatory system.
➤ To delineate the normal parameters and potential pathological variations in fetal heart rate (FHR), including baseline FHR, variability, accelerations, and decelerations, as essential indicators of fetal well-being.
➤ To familiarize oneself with the methodologies and interpretations of fetal well-being assessments, such as the Non-Stress Test (NST) and Biophysical Profile (BPP).
➤ To articulate the profound physiological transformations that occur within the cardiovascular system during the transition from intrauterine to extrauterine life, specifically focusing on the closure of fetal shunts and the establishment of independent cardiopulmonary function.
➤ To integrate theoretical knowledge with practical nursing interventions for monitoring and managing maternal-newborn conditions related to fetal circulation.
Introduction
Fetal circulation represents a highly specialized cardiovascular system meticulously designed to support fetal development within the intrauterine environment, where oxygenation and nutrient exchange are primarily mediated by the placenta rather than the immature pulmonary and gastrointestinal systems.
This unique circulatory configuration involves a series of anatomical shunts that strategically divert blood flow, ensuring that vital organs such as the brain and myocardium receive preferentially oxygenated blood while bypassing the non-functional lungs and partially functional liver. The fundamental distinctions between fetal and adult circulation are imperative for comprehending the physiological adaptations essential for successful extrauterine transition.
Postnatally, the dramatic shifts in oxygen tension, systemic vascular resistance, and pulmonary vascular resistance orchestrate the closure of these fetal shunts, thereby establishing the mature, sequential circulatory pattern characteristic of independent life.
A comprehensive understanding of these mechanisms is paramount for nursing professionals to accurately assess fetal well-being, anticipate potential complications, and implement timely and effective interventions during both antenatal and immediate postnatal periods.
Fetal Circulation: Anatomic And Physiologic Considerations
Fetal circulation is an intricate system specifically adapted for intrauterine life, where the placenta serves as the organ for gas exchange, nutrient delivery, and waste removal. This system differs significantly from postnatal circulation due to the non-functional fetal lungs and the partially functional fetal liver, necessitating several shunts to direct blood flow appropriately.
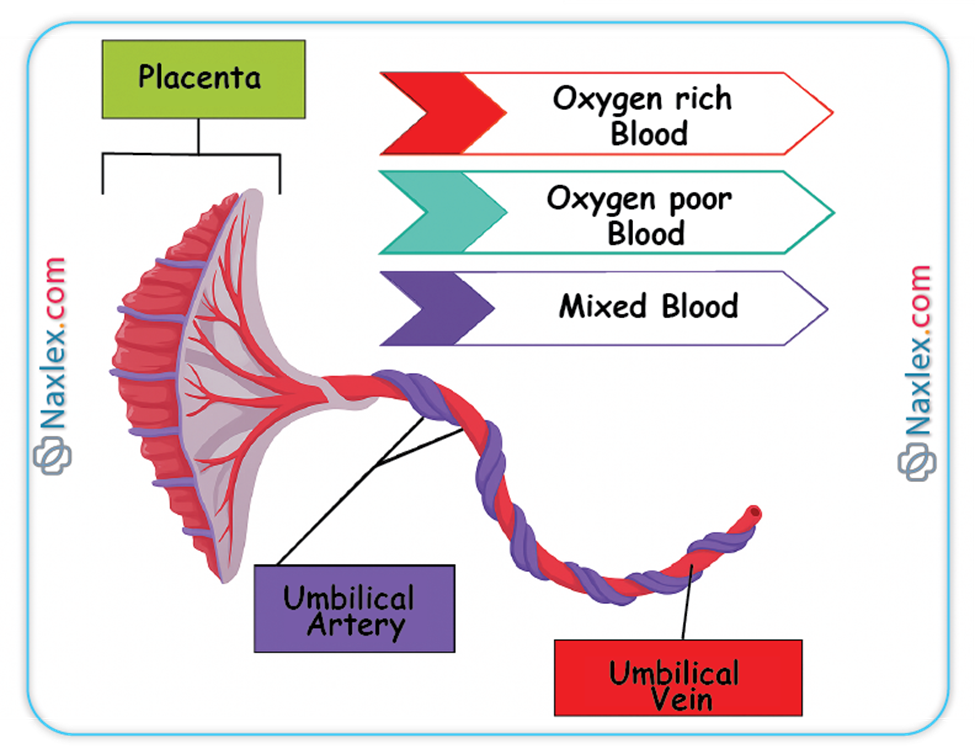
Umbilical Cord
The umbilical cord serves as the vital conduit connecting the fetus to the placenta, facilitating all essential exchanges between the maternal and fetal circulations.
➤ Gross Anatomy
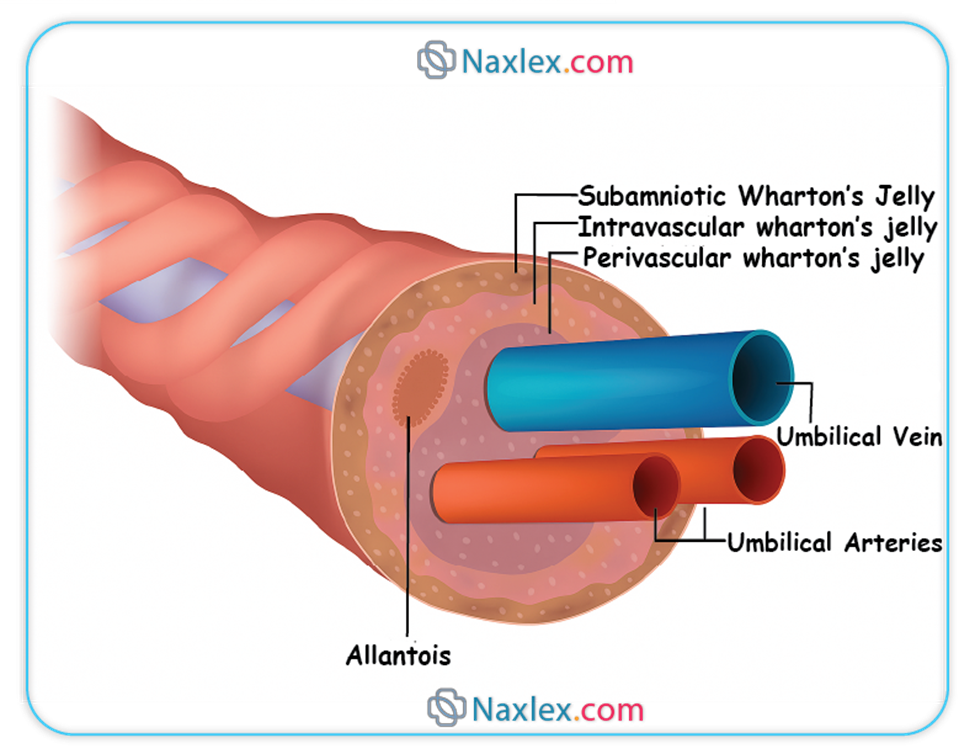
◆ The umbilical cord typically measures approximately 50 to 60 centimeters in length and 1 to 2 centimeters in diameter at term gestation.
◆ It is characterized by a helical twisting pattern, which is thought to provide mechanical strength and protect the vessels within.
◆ The cord is encased in Wharton's jelly, a specialized mucopolysaccharide matrix that provides structural support and protection to the enclosed blood vessels, preventing compression and kinking.
◆ The absence of Wharton's jelly or an excessively long or short cord can predispose to complications such as cord prolapse or compression, thereby compromising fetal perfusion.
➤ Microscopic Anatomy
◆ The umbilical cord characteristically contains three distinct vascular structures: two umbilical arteries and one umbilical vein.
◆ Umbilical Vein: This singular vessel carries oxygenated and nutrient-rich blood from the chorionic villi of the placenta directly to the fetal circulation. Its oxygen saturation is remarkably high for fetal blood, typically ranging from 80-85%.
◆ Umbilical Arteries: These paired vessels transport deoxygenated blood and metabolic waste products from the fetus back to the placenta for maternal excretion. These arteries originate from the internal iliac arteries of the fetus.
◆ Nursing Insights: The presence of only two vessels (a single umbilical artery, SUA) is an anomaly occurring in approximately 1% of pregnancies and may be associated with other congenital anomalies, particularly renal or cardiac defects. Therefore, a meticulous assessment of the umbilical cord at birth is crucial for early detection and further diagnostic evaluation.
Placental Functions in Fetal Circulation
The placenta is a multifaceted organ critical for sustaining fetal life, acting as the primary interface between maternal and fetal systems.
➤ The placenta serves as the fetal "lungs," facilitating efficient gas exchange (oxygen from mother to fetus, carbon dioxide from fetus to mother) via diffusion. This process is optimized by the higher oxygen affinity of fetal hemoglobin (HbF) compared to adult hemoglobin (HbA).
➤ It acts as the fetal "gastrointestinal tract," enabling the transfer of nutrients (e.g., glucose, amino acids, fatty acids, vitamins) from maternal blood to fetal blood through active transport and facilitated diffusion.
➤ The placenta functions as the fetal "kidneys," removing metabolic waste products (e.g., urea, creatinine) from fetal blood and transferring them to the maternal circulation for excretion.
➤ It plays a vital role in hormone production (e.g., human chorionic gonadotropin [hCG], progesterone, estrogen), which are essential for maintaining pregnancy and influencing fetal development.
➤ The placenta also provides immunological protection by transferring maternal antibodies (IgG) to the fetus, conferring passive immunity against various pathogens.
➤ Nursing Insights: Any compromise to placental function, such as placental abruption, placenta previa, or uteroplacental insufficiency, directly impairs fetal oxygenation and nutrient delivery, leading to conditions like intrauterine growth restriction (IUGR) or fetal distress. Monitoring maternal blood pressure and assessing for signs of hemorrhage are paramount for early identification of these critical conditions.
Fetal Circulatory Shunts
In utero, the fetal lungs are fluid-filled and offer high vascular resistance, making them largely non-functional for gas exchange. Similarly, the fetal liver is still developing and does not require full perfusion. To circumvent these organs, specialized vascular shunts are present, which largely close after birth.
➤ Ductus Venosus
◆ The ductus venosus is a vascular shunt that allows a significant portion of the oxygenated blood carried by the umbilical vein to bypass the hepatic circulation and flow directly into the inferior vena cava.
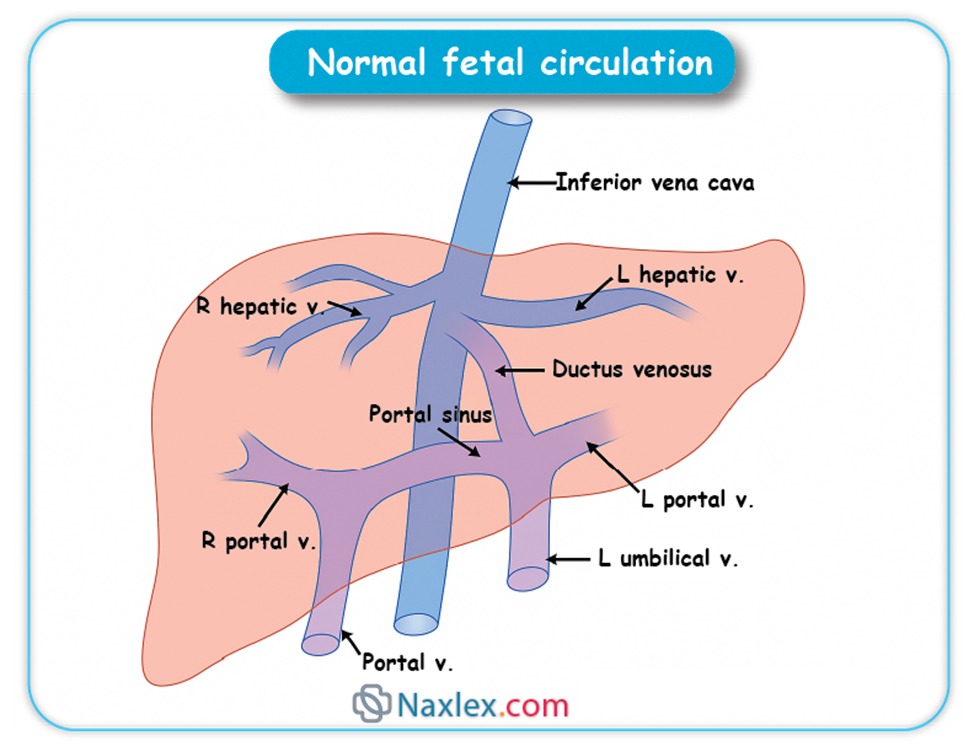
◆ Approximately 50% to 75% of the highly oxygenated blood from the umbilical vein is shunted through the ductus venosus, directly delivering it to the heart.
◆ This preferential shunting ensures that the most oxygen-rich blood reaches the heart and subsequently the fetal brain and myocardium.
◆ Nursing Insights: Patency of the ductus venosus is crucial for adequate fetal oxygenation. Persistent patency postnatally is rare but can be associated with congenital heart defects or liver anomalies.
➤ Foramen Ovale
◆ The foramen ovale is an oval-shaped opening located in the interatrial septum, connecting the right atrium directly to the left atrium.
◆ Due to the high pulmonary vascular resistance and relatively low systemic vascular resistance in the fetus, blood pressure in the right atrium is higher than in the left atrium.
◆ This pressure gradient facilitates the flow of preferentially oxygenated blood from the inferior vena cava, entering the right atrium, across the foramen ovale into the left atrium.
◆ From the left atrium, this blood flows into the left ventricle and is then ejected into the ascending aorta, supplying the fetal brain and upper extremities with highly oxygenated blood.
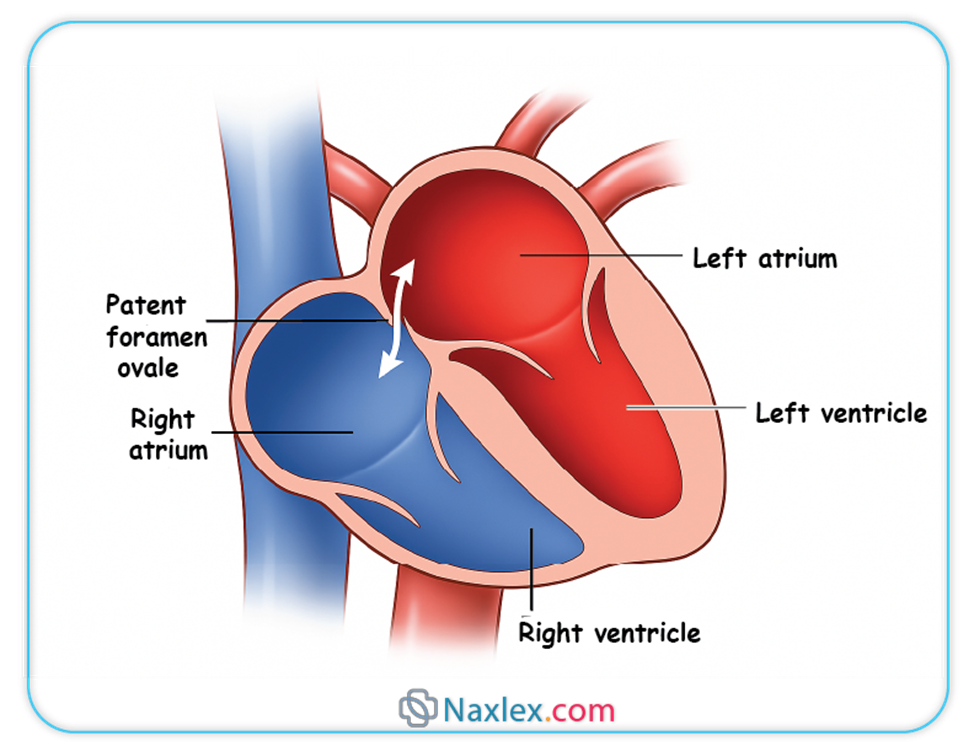
◆ Nursing Insights: The foramen ovale allows the majority of blood to bypass the non-functional fetal lungs. Failure of the foramen ovale to functionally close after birth, leading to a patent foramen ovale (PFO), is common and often asymptomatic, but can rarely be associated with paradoxical emboli or certain types of stroke in adults.
➤ Ductus Arteriosus
◆ The ductus arteriosus is a vascular connection between the pulmonary artery and the aorta.
◆ Due to the high pulmonary vascular resistance, only a small amount of blood flows to the fetal lungs for nutritional purposes. The majority of blood ejected from the right ventricle into the pulmonary artery is shunted through the ductus arteriosus directly into the descending aorta.
◆ This shunt ensures that the pulmonary circulation is largely bypassed, directing deoxygenated blood from the right ventricle into the systemic circulation, primarily supplying the lower extremities and returning to the placenta via the umbilical arteries.
◆ Prostaglandin E2 (PGE2), produced by the placenta and the ductus arteriosus itself, plays a crucial role in maintaining the patency of this shunt in utero.
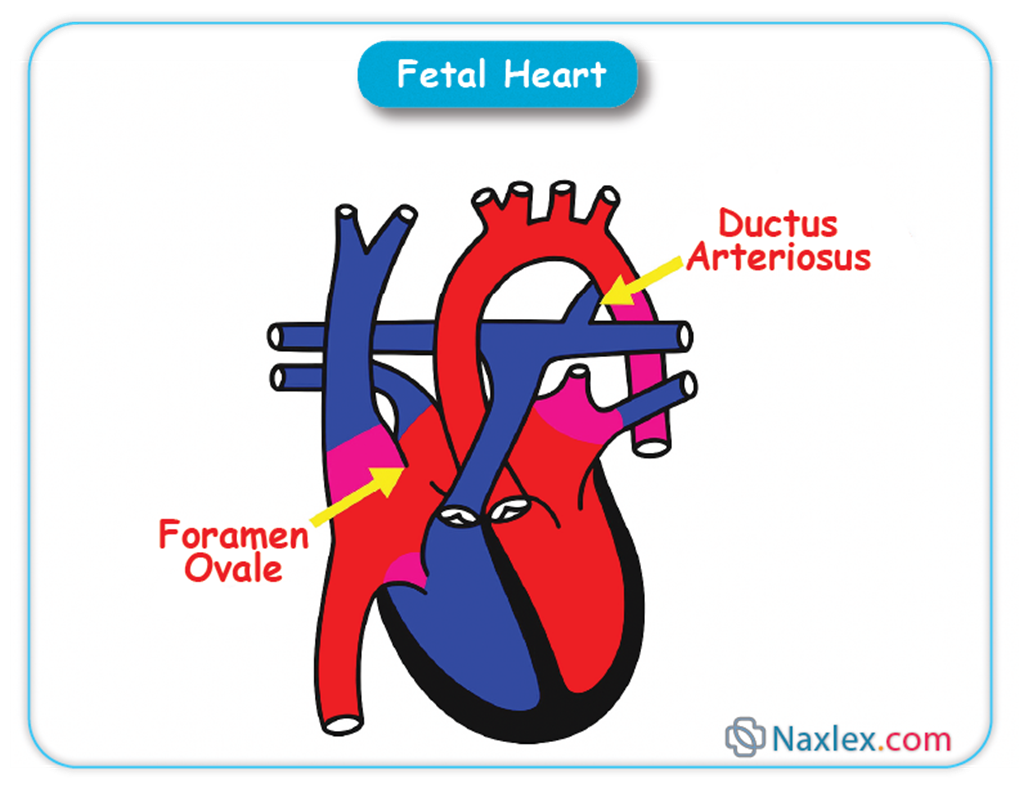
◆ Nursing Insights: The ductus arteriosus is vital for diverting blood away from the fetal lungs. A persistent patent ductus arteriosus (PDA) after birth can lead to left-to-right shunting, resulting in pulmonary overcirculation, heart failure, and respiratory distress. Pharmacological interventions (e.g., indomethacin) or surgical ligation may be required to close a symptomatic PDA.
Fetal Hemoglobin (HbF)
Fetal hemoglobin (HbF) is a specialized form of hemoglobin that plays a crucial role in facilitating oxygen transport within the intrauterine environment.
➤ HbF possesses a significantly higher affinity for oxygen than adult hemoglobin (HbA). This is attributed to its different subunit composition (two alpha and two gamma chains) and a reduced binding affinity for 2,3-bisphosphoglycerate (2,3-BPG).
➤ This enhanced oxygen affinity enables HbF to efficiently extract oxygen from the maternal circulation across the placental membrane, even at lower partial pressures of oxygen. This ensures adequate oxygenation of fetal tissues despite the relatively hypoxic intrauterine environment compared to extrauterine life.
➤ Nursing Insights: The unique properties of HbF are essential for fetal survival. Postnatally, HbF is gradually replaced by HbA. Understanding this transition is critical for assessing neonatal oxygenation and identifying conditions like neonatal anemia or polycythemia, which may impact oxygen carrying capacity.
Pathways of Blood Flow in Fetal Circulation
The flow of blood in the fetus is precisely orchestrated to ensure optimal oxygen and nutrient delivery to developing organs.
➤ Highly oxygenated blood from the placenta travels via the umbilical vein to the fetus.
➤ Upon entering the fetal abdomen, the umbilical vein partially branches to perfuse the liver, but a substantial portion bypasses the hepatic circulation via the ductus venosus, directly joining the inferior vena cava.
➤ Blood in the inferior vena cava, now a mixture of oxygenated blood from the umbilical vein and deoxygenated blood from the fetal lower body, enters the right atrium.
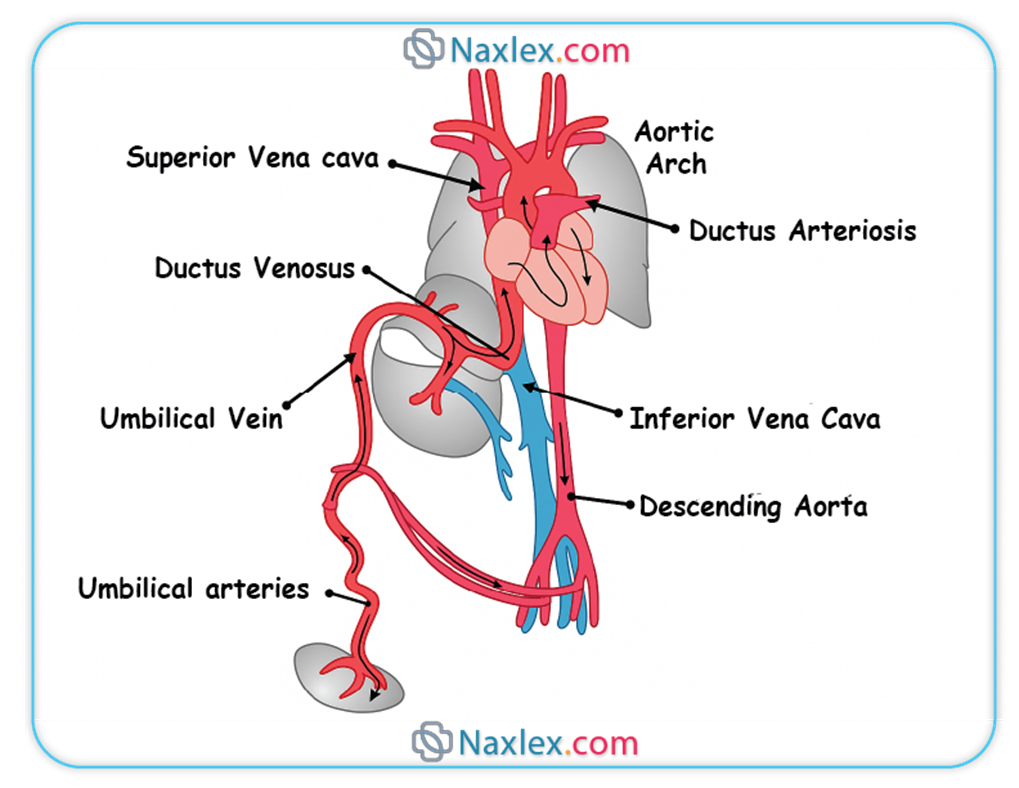
➤ In the right atrium, the majority of this blood is shunted through the foramen ovale into the left atrium, largely bypassing the pulmonary circulation due to high pulmonary vascular resistance.
➤ From the left atrium, blood flows into the left ventricle and is then pumped into the ascending aorta, primarily supplying the brain, myocardium, and upper extremities with relatively high oxygen content.
➤ Deoxygenated blood from the fetal upper body and head returns to the right atrium via the superior vena cava. This blood, along with a small amount of blood from the inferior vena cava that did not cross the foramen ovale, enters the right ventricle.
➤ The right ventricle pumps this blood into the pulmonary artery. Due to high pulmonary vascular resistance, only a small percentage of blood flows to the lungs. The majority is shunted through the ductus arteriosus into the descending aorta.
➤ The descending aorta carries mixed blood (less oxygenated than that in the ascending aorta) to the lower extremities and abdominal organs.
➤ Finally, deoxygenated blood and waste products return to the placenta via the two umbilical arteries (branches of the internal iliac arteries) for re-oxygenation and waste removal.
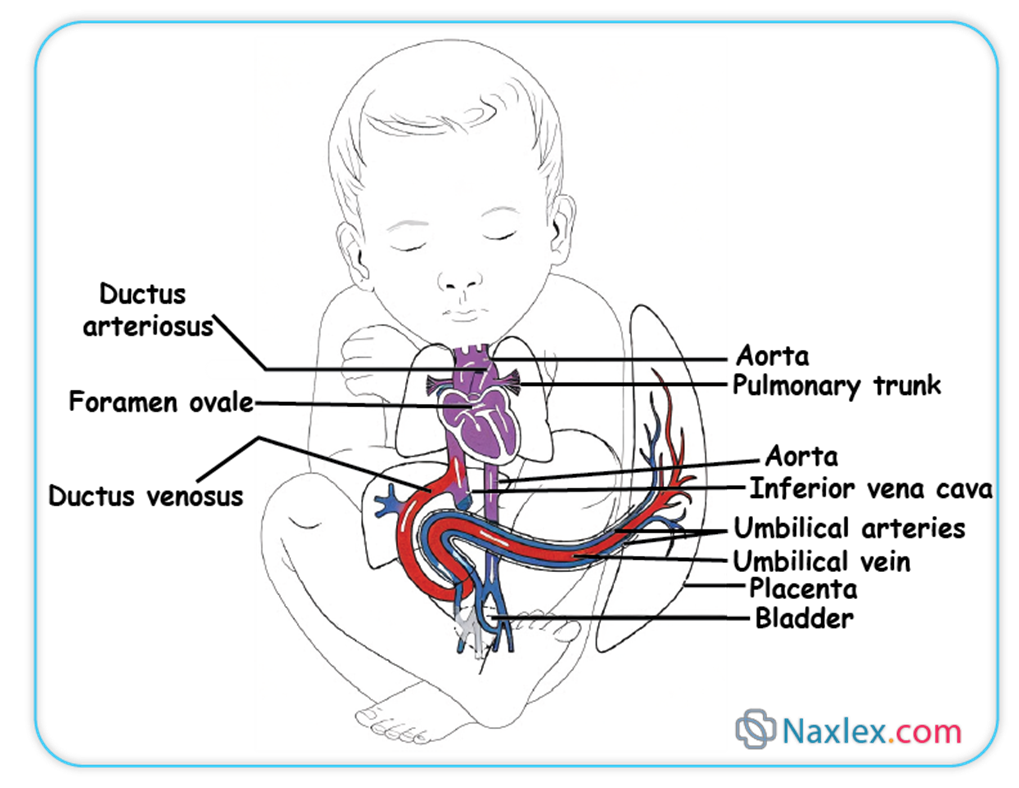
Nursing Insights: A thorough understanding of this pathway is fundamental for interpreting the significance of various congenital heart defects. For instance, transposition of the great arteries necessitates communication between systemic and pulmonary circulations (e.g., a patent ductus arteriosus or foramen ovale) to sustain life.
Fetal Heart Rate (fhr) Monitoring
Fetal heart rate (FHR) monitoring is an essential clinical tool utilized to assess fetal oxygenation status and overall well-being, particularly during labor and in high-risk pregnancies. The interpretation of FHR patterns provides critical insights into the adequacy of oxygen supply to the fetal brain and myocardium.
Baseline FHR
The baseline fetal heart rate represents the approximate mean FHR observed during a 10-minute segment, excluding accelerations, decelerations, and periods of marked variability.
➤ The normal range for baseline FHR in a term fetus is 110 to 160 beats per minute (bpm).
➤ Fetal Tachycardia is defined as a baseline FHR greater than 160 bpm for a duration of 10 minutes or longer.
◆ Common causes include: maternal fever, maternal dehydration, maternal hyperthyroidism, chorioamnionitis, fetal hypoxia, fetal infection, maternal illicit drug use (e.g., cocaine, methamphetamines), and certain maternal medications (e.g., beta-agonists).
◆ Nursing Insights: Persistent fetal tachycardia warrants immediate investigation to identify and address the underlying cause. Interventions may include antipyretics for maternal fever, intravenous fluid administration for dehydration, and close monitoring for signs of fetal distress.
➤ Fetal Bradycardia is defined as a baseline FHR less than 110 bpm for a duration of 10 minutes or longer.
◆ Common causes include: prolonged maternal hypotension, umbilical cord compression, fetal hypoxia, maternal hypothermia, maternal hypoglycemia, maternal beta-blocker administration, and complete heart block in the fetus.
◆ Nursing Insights: Fetal bradycardia can be a significant indicator of fetal distress and requires urgent intervention. Nursing actions may include repositioning the mother, administering supplemental oxygen, increasing intravenous fluid rate, and notifying the healthcare provider promptly.
FHR Variability
FHR variability refers to the fluctuations in the baseline FHR, reflecting the interplay between the sympathetic and parasympathetic branches of the fetal autonomic nervous system. It is considered the most significant indicator of fetal well-being and neurological integrity.
➤ Absent Variability: Amplitude range undetectable. This is a highly concerning finding, suggesting severe fetal hypoxia, metabolic acidemia, neurological compromise, or the effect of central nervous system depressant medications.
➤ Minimal Variability: Amplitude range detectable but 5 bpm or less. This can be caused by fetal sleep cycles (typically lasting 20-40 minutes), prematurity, maternal medications (e.g., opioids, magnesium sulfate), or mild hypoxia. If persistent and not attributable to sleep cycles, it is a concerning sign.
➤ Moderate Variability: Amplitude range 6 to 25 bpm. This is considered the most reassuring FHR pattern, indicating a well-oxygenated fetus with an intact and responsive autonomic nervous system.
➤ Marked Variability: Amplitude range greater than 25 bpm. This can be an early sign of fetal hypoxia, often seen during transient umbilical cord compression or fetal stimulation. However, it can also be associated with sympathetic nervous system hyperactivity. If persistent, it warrants further evaluation.
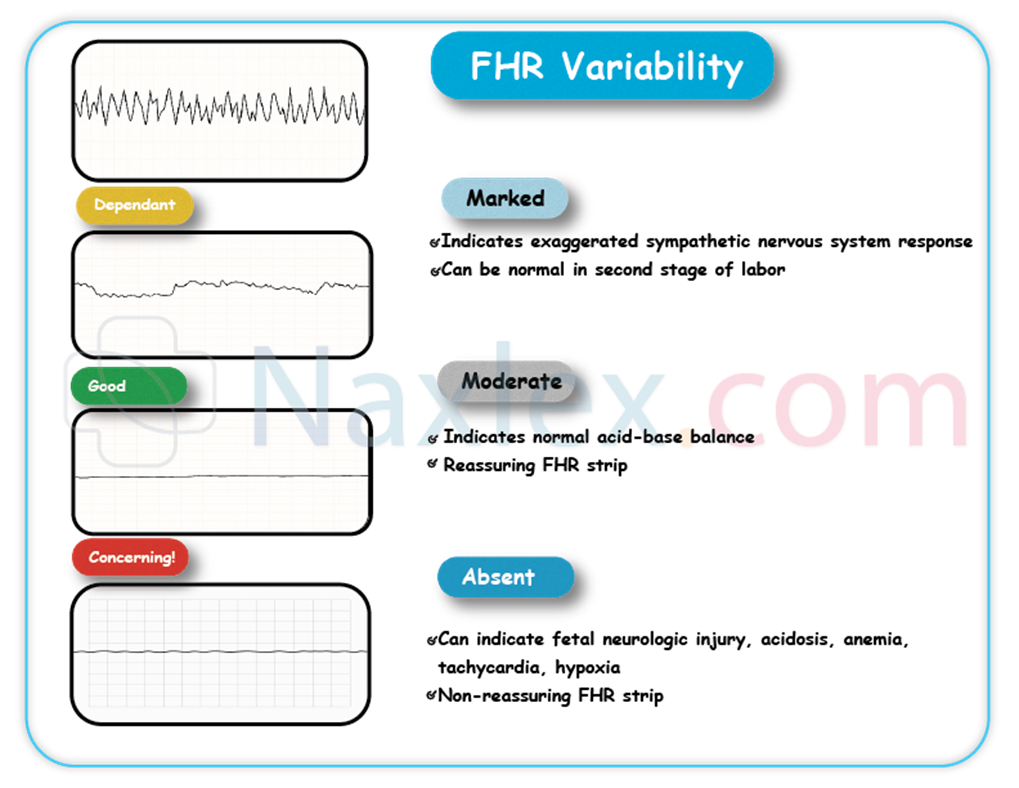
➤ Nursing Insights: Continuous assessment of FHR variability is critical. A change from moderate to minimal or absent variability, especially when associated with decelerations, necessitates immediate intervention. Conversely, the presence of moderate variability is a strong indicator of fetal well-being. Nursing interventions for concerning variability often involve intrauterine resuscitation measures.
FHR Accelerations
Accelerations are transient increases in the fetal heart rate above the baseline. They are considered a reassuring sign of fetal well-being.
➤ In a term fetus, an acceleration is defined as an abrupt increase in FHR (onset to peak in <30 seconds) of at least 15 bpm above the baseline, lasting for at least 15 seconds but less than 2 minutes.
➤ In a preterm fetus (<32 weeks gestation), an acceleration is defined as an abrupt increase of at least 10 bpm above the baseline, lasting for at least 10 seconds.
➤ The presence of accelerations typically indicates an intact and reactive fetal nervous system and adequate fetal oxygenation. They often occur in response to fetal movement or external stimulation.
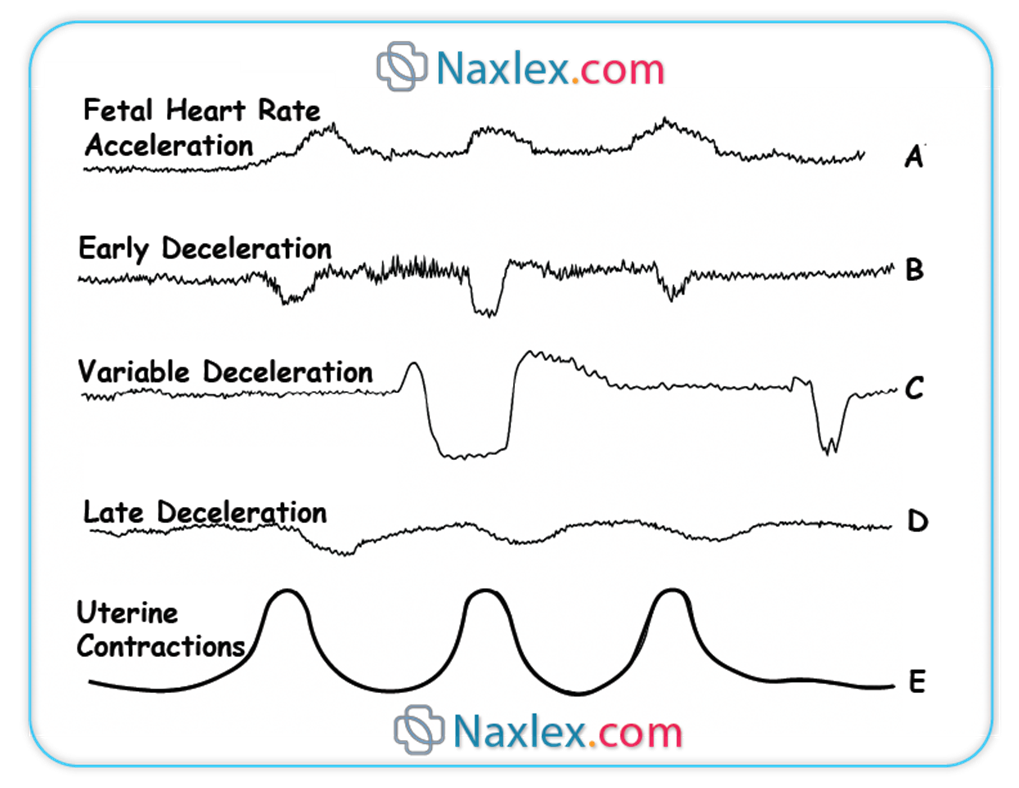
➤ Nursing Insights: Accelerations are a positive finding and contribute to a reactive Non-Stress Test (NST). Their absence in an otherwise normal tracing may warrant further evaluation to rule out fetal sleep cycles or other factors.
FHR Decelerations
Decelerations are transient decreases in the fetal heart rate below the baseline. Their classification and characteristics provide crucial information regarding the potential etiology and fetal status.
➤ Early Decelerations
◆ Early decelerations are characterized by a gradual decrease in FHR and a return to baseline that is visually symmetrical with the uterine contraction. The nadir of the deceleration coincides with the peak of the contraction.
◆ They are typically benign and are caused by fetal head compression during uterine contractions, leading to vagal nerve stimulation.
◆ Nursing Insights: Early decelerations are generally considered normal and do not require intervention. Documentation and continued monitoring are appropriate.
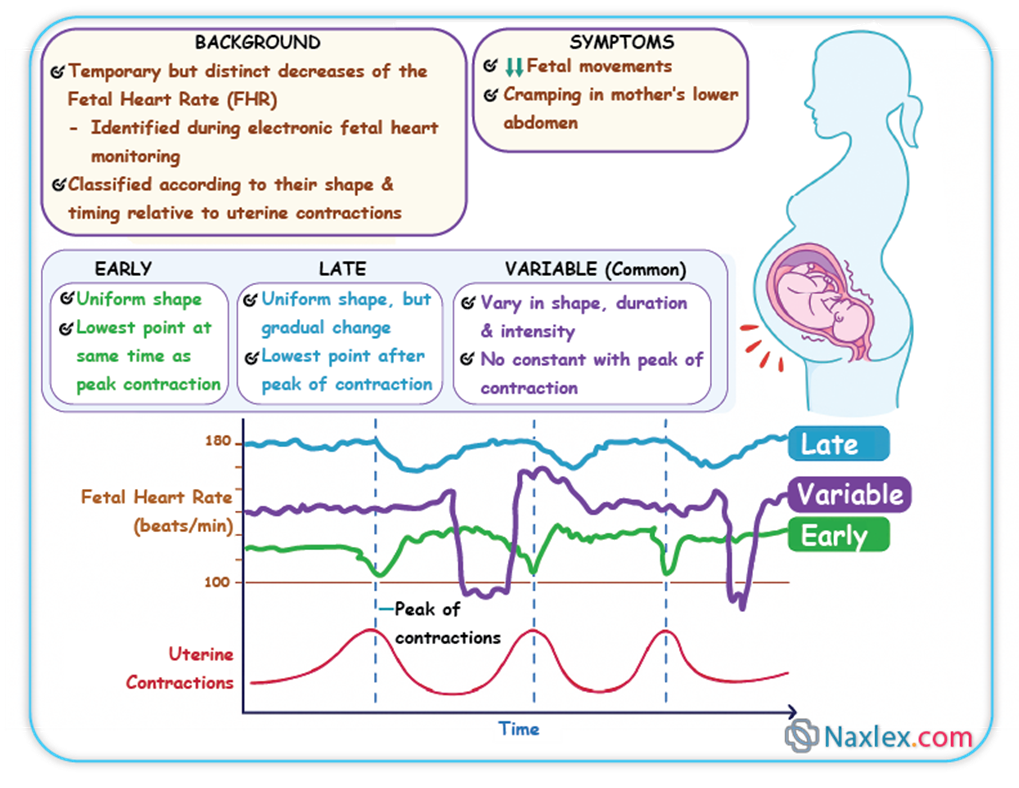
➤ Late Decelerations
◆ Late decelerations are characterized by a gradual decrease in FHR and a return to baseline that occurs after the peak of the uterine contraction, with the nadir of the deceleration occurring after the peak of the contraction.
◆ They are indicative of uteroplacental insufficiency, meaning a reduction in blood flow and oxygen transfer from the placenta to the fetus, often due to maternal hypotension, uterine hypertonus, or placental pathologies.
◆ Late decelerations are a concerning sign and suggest fetal hypoxia.
◆ Nursing Insights: Prompt nursing interventions are essential for late decelerations. These include: repositioning the mother (left lateral or right lateral), administering supplemental oxygen via face mask, increasing intravenous fluid rate, discontinuing oxytocin if infusing, notifying the healthcare provider, and preparing for potential delivery if the pattern persists despite interventions.
➤ Variable Decelerations
◆ Variable decelerations are characterized by an abrupt decrease in FHR (onset to nadir <30 seconds), with a variable shape, duration, and degree of fall. They often appear "V," "W," or "U" shaped and do not consistently correlate with uterine contractions.
◆ They are most commonly caused by umbilical cord compression, leading to transient alterations in umbilical blood flow. This can be due to cord prolapse, nuchal cord, or oligohydramnios.
◆ While often benign if infrequent and shallow, recurrent or prolonged variable decelerations can lead to fetal hypoxia and acidosis.
◆ Nursing Insights: Interventions for variable decelerations often include: repositioning the mother, administering supplemental oxygen, increasing intravenous fluid rate, and considering amnioinfusion if severe and recurrent (to relieve cord compression). Close monitoring for changes in variability or progression to other concerning patterns is crucial.
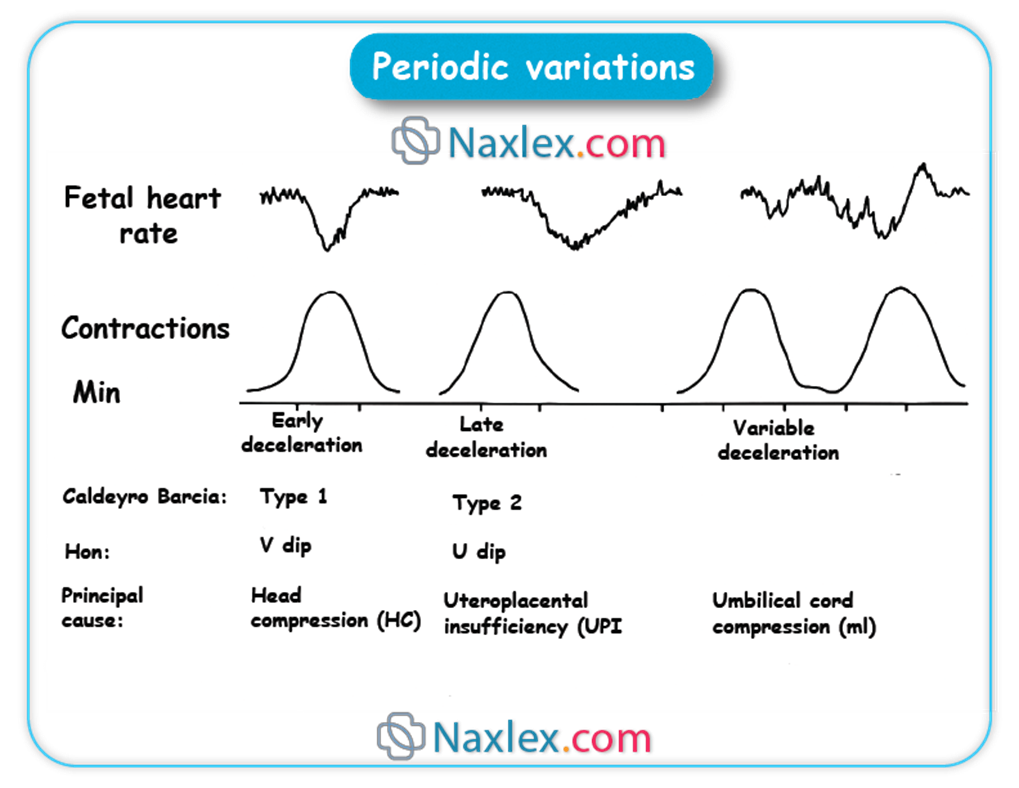
➤ Prolonged Decelerations
◆ A prolonged deceleration is a decrease in FHR of at least 15 bpm below the baseline, lasting for 2 minutes or more but less than 10 minutes.
◆ Causes can include: sustained umbilical cord compression, maternal hypotension, uterine tachysystole, placental abruption, uterine rupture, or prolonged maternal seizure.
◆ Nursing Insights: A prolonged deceleration is an urgent situation. Immediate interventions are required to identify and correct the underlying cause. If the deceleration persists for 10 minutes or longer, it is classified as a change in baseline FHR (bradycardia) and is a medical emergency often requiring immediate delivery.
Practice Exercise 2
- Central Sensitization: This refers to the increased sensitivity and hyperexcitability of neurons within the CNS. It can be caused by peripheral tissue damage or nerve injury and can lead to significant pain from normally non-painful stimuli (allodynia) and increased pain responses to noxious stimuli (hyperalgesia). Central sensitization can lead to chronic pain even after the initial injury has healed.
- Windup: A temporary increase in the firing of dorsal horn neurons due to ongoing stimulation of C-fibers, which depends on the activation of NMDA receptors. NMDA receptor antagonists like ketamine can interrupt this process.
- Neuroplasticity: This is the brain's ability for neurons to compensate for injury and adjust responses to new situations. While it can contribute to reducing pain, it can also lead to maladaptive mechanisms that enhance pain. Genetic differences can influence individual responses to pain and the development of chronic pain.
- Clinical Manifestations of Central Sensitization:
- Hyperalgesia: Increased pain response to noxious stimuli.
- Allodynia: Painful responses to normally innocuous (harmless) stimuli.
- Persistent Pain: Prolonged pain even after the original noxious stimulus ends.
- Secondary Hyperalgesia: Extension of tenderness or increased pain sensitivity beyond the area of injury into uninjured tissue.
Fetal Well-being Assessment
Fetal well-being assessment refers to various diagnostic tests and monitoring techniques employed to evaluate the health, oxygenation, and overall condition of the fetus, particularly in pregnancies complicated by risk factors or concerns about fetal compromise.
Non-Stress Test (NST)
The Non-Stress Test (NST) is a non-invasive antepartum test that evaluates fetal well-being by monitoring fetal heart rate accelerations in response to spontaneous fetal movement.
➤ Procedure: The mother is placed in a semi-Fowler's or left lateral position to prevent supine hypotension. External fetal heart rate and uterine activity transducers are applied. The FHR is monitored for a minimum of 20 minutes, observing for accelerations.
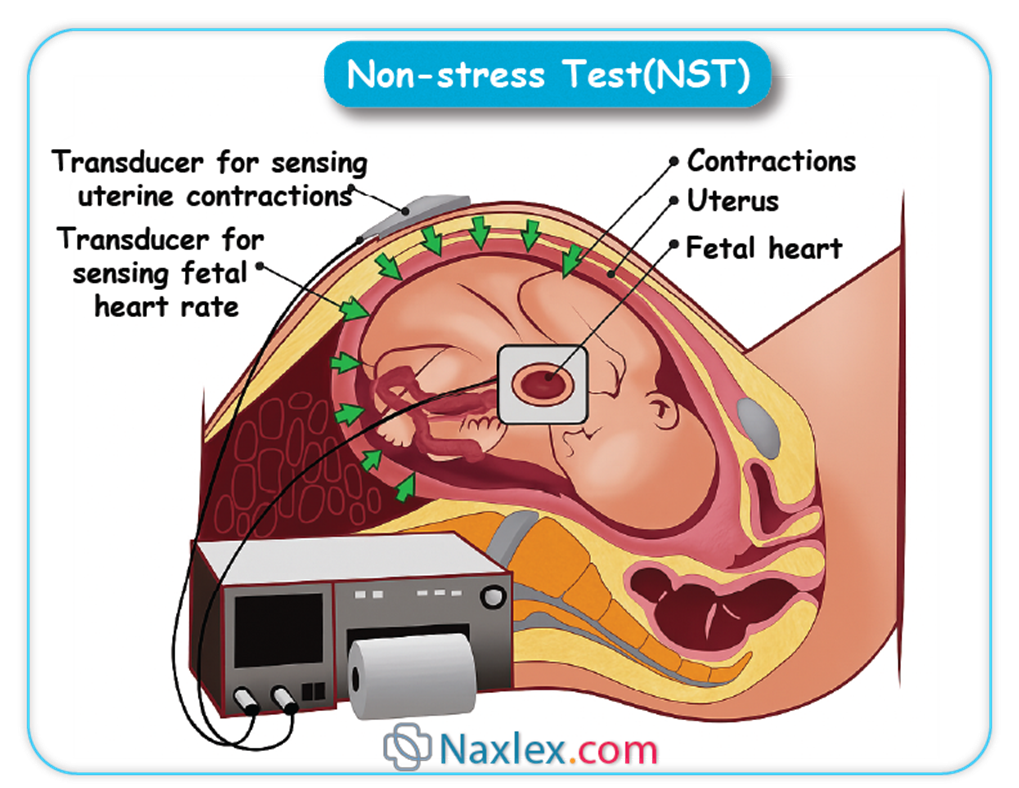
➤ Interpretation:
◆ Reactive NST (Reassuring): This indicates fetal well-being. A reactive NST is characterized by at least two FHR accelerations (meeting criteria for gestational age: ≥15 bpm above baseline, lasting ≥15 seconds in a term fetus; ≥10 bpm above baseline, lasting ≥10 seconds in a preterm fetus) within a 20-minute period. Fetal movement may or may not be perceived by the mother.
◆ Nonreactive NST (Non-reassuring): This indicates that the criteria for a reactive NST have not been met within a 20-minute period. A nonreactive NST may be due to a fetal sleep cycle, maternal medications, or fetal hypoxemia.
◆ Unsatisfactory NST: This occurs when the quality of the FHR tracing is inadequate for interpretation.
➤ Nursing Insights: A nonreactive NST necessitates further evaluation, which may include extending the monitoring period to 40 minutes to account for fetal sleep cycles, or proceeding to a Biophysical Profile (BPP) or Contraction Stress Test (CST) to further assess fetal status. Patient education regarding the purpose and interpretation of the NST is essential.
Biophysical Profile (BPP)
The Biophysical Profile (BPP) is a comprehensive assessment of fetal well-being that combines a Non-Stress Test (NST) with a real-time ultrasound examination to evaluate five specific biophysical parameters.
➤ Components of a BPP: Each component is scored 0 (absent or abnormal) or 2 (present or normal), with a maximum total score of 10.
◆ Fetal Breathing Movements: At least one episode of rhythmic breathing movements of ≥30 seconds duration within 30 minutes. (Score 2: Present; Score 0: Absent or <30 seconds)
◆ Gross Fetal Body Movements: At least three discrete body or limb movements within 30 minutes. (Score 2: Present; Score 0: <3 movements)
◆ Fetal Tone: At least one episode of active extension with return to flexion of a limb or trunk, or opening and closing of a hand. (Score 2: Present; Score 0: Absent or slow extension/flexion)
◆ Reactive Fetal Heart Rate (NST): As defined above (at least two accelerations within 20 minutes). (Score 2: Reactive; Score 0: Nonreactive)
◆ Amniotic Fluid Volume (AFV): A single deepest pocket of amniotic fluid measuring ≥2 cm in two perpendicular planes, or an Amniotic Fluid Index (AFI) ≥5 cm. (Score 2: Adequate; Score 0: Inadequate/Oligohydramnios)
➤ Interpretation of BPP Scores:
◆ Score 8-10 (Normal): Reassuring for fetal well-being.
◆ Score 6 (Equivocal): May indicate potential fetal compromise, especially if oligohydramnios is present. Further evaluation or repeat BPP within 24 hours may be indicated.
◆ Score ≤4 (Abnormal): Highly suggestive of fetal compromise. Clinical management often involves consideration for immediate delivery.
➤ Nursing Insights: The BPP provides a more comprehensive assessment of fetal health than the NST alone. Nurses play a crucial role in preparing the patient, assisting with the ultrasound, and educating the patient about the results and subsequent plan of care. Understanding the significance of each component allows for appropriate clinical judgment. For instance, absent breathing movements and poor tone can be early indicators of developing hypoxia.
Transition From Fetal To Neonatal Circulation
The transition from fetal to neonatal circulation is one of the most remarkable physiological adaptations occurring at birth, involving profound cardiovascular and pulmonary changes that enable independent extrauterine life. This process involves the cessation of placental blood flow, the initiation of pulmonary respiration, and the functional and anatomical closure of the fetal circulatory shunts.
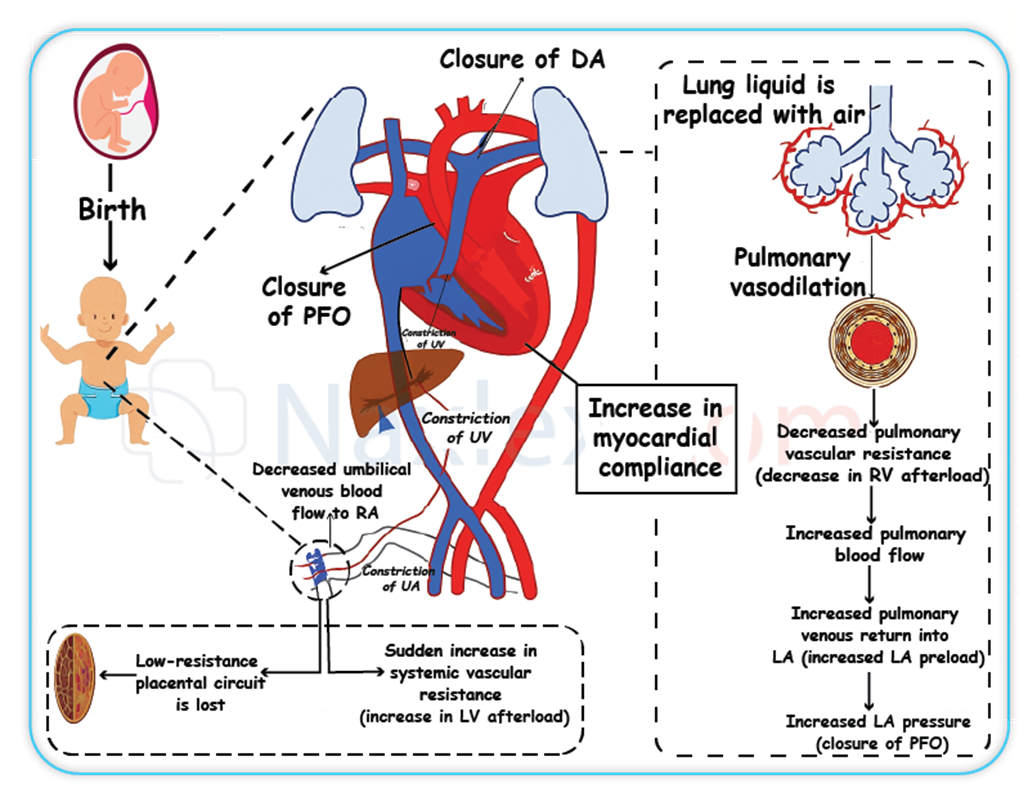
Physiological Changes at Birth
The initiation of respiration and the clamping of the umbilical cord trigger a cascade of events that fundamentally alter the circulatory dynamics.
➤ Lung Expansion and Decrease in Pulmonary Vascular Resistance (PVR):
◆ Upon the first breath, the infant's lungs expand, replacing the fetal lung fluid with air. This expansion significantly decreases the normally high pulmonary vascular resistance (PVR).
◆ Simultaneously, the increase in oxygen concentration in the pulmonary arterioles causes vasodilation, further reducing PVR.
◆ This dramatic reduction in PVR leads to a significant increase in pulmonary blood flow, allowing the lungs to take over the role of gas exchange.
➤ Increase in Systemic Vascular Resistance (SVR):
◆ Clamping of the umbilical cord removes the low-resistance placental circulation from the systemic circuit. This immediately results in a significant increase in systemic vascular resistance (SVR) throughout the body.
◆ The combined effect of decreased PVR and increased SVR reverses the pressure gradients that maintained fetal shunts.
➤ Changes in Atrial Pressures:
◆ With increased pulmonary blood flow, more blood returns to the left atrium from the pulmonary veins, leading to a rise in left atrial pressure.
◆ Concurrently, the cessation of umbilical venous return to the right atrium leads to a decrease in right atrial pressure.
◆ This reversal of pressure gradient (left atrial pressure > right atrial pressure) is crucial for the closure of the foramen ovale.
➤ Nursing Insights: Monitoring the newborn's respiratory effort, heart rate, and oxygen saturation immediately after birth is paramount for assessing the success of this circulatory transition. Delayed or inadequate transition can manifest as persistent pulmonary hypertension of the newborn (PPHN) or cyanotic congenital heart disease.
Closure of Fetal Shunts and Vessels
The altered pressure gradients and increased oxygenation initiate the functional and, subsequently, anatomical closure of the fetal shunts and umbilical vessels, transforming the circulatory system to an adult pattern.
➤ Ductus Venosus
◆ Functional Closure: Occurs shortly after birth, typically within minutes to hours, due to the cessation of umbilical venous flow and mechanical compression.
◆ Anatomical Closure: Fibrosis occurs gradually over several days to weeks.
◆ Remnant: Becomes the ligamentum venosum.
◆ Nursing Insights: While generally a rapid closure, persistent patency is rare but would divert portal venous blood away from the liver, potentially affecting hepatic function.
➤ Foramen Ovale
◆ Functional Closure: Occurs almost immediately at birth due to the increased left atrial pressure and decreased right atrial pressure. The flap-like valve of the foramen ovale is pressed against the interatrial septum, effectively closing the shunt.
◆ Anatomical Closure: Complete anatomical fusion typically occurs over several weeks to months, potentially up to a year, leading to the formation of the fossa ovalis.
◆ Nursing Insights: A probe-patent foramen ovale (PFO), where anatomical closure is not complete but functional closure usually maintains, is found in a significant percentage of adults (up to 25%). While often asymptomatic, under certain conditions (e.g., Valsalva maneuver), transient right-to-left shunting can occur.
➤ Ductus Arteriosus
◆ Functional Closure: Begins rapidly after birth, usually within 10-15 hours, primarily due to the rise in systemic oxygen tension and the decrease in circulating prostaglandin E2 (PGE2) levels (as the placenta, a major source of PGE2, is removed). The increased oxygen levels cause vasoconstriction of the ductus arteriosus.
◆ Anatomical Closure: Fibrosis of the vessel wall takes approximately 2-3 weeks to complete, forming the ligamentum arteriosum.
◆ Nursing Insights: Failure of the ductus arteriosus to functionally close results in a Patent Ductus Arteriosus (PDA), a common congenital heart defect, particularly in premature infants. A PDA leads to left-to-right shunting, causing pulmonary overcirculation and potentially pulmonary hypertension or heart failure. Clinical signs include a continuous "machinery-like" murmur, bounding pulses, and signs of respiratory distress. Medical management with prostaglandin inhibitors (e.g., indomethacin, ibuprofen) or surgical ligation may be required.
➤ Umbilical Vessels
◆ Umbilical Arteries: After the umbilical cord is clamped, the umbilical arteries constrict and functionally close within minutes, preventing blood loss from the newborn.
◆ Remnant: Anatomical obliteration occurs over 2-3 months, forming the medial umbilical ligaments.
◆ Umbilical Vein: Functionally closes with the cessation of umbilical blood flow.
◆ Remnant: Anatomically obliterates to become the ligamentum teres (round ligament of the liver).
◆ Nursing Insights: Prompt assessment of the umbilical cord for the presence of two arteries and one vein is standard practice upon birth. Delayed clamping of the umbilical cord is now often advocated to allow for maximal blood transfer from the placenta to the infant, increasing iron stores and reducing the risk of anemia.
Summary Of Concepts
Fetal circulation is a sophisticated circulatory system uniquely adapted for intrauterine existence, characterized by the placenta as the primary organ for gas exchange and nutrient transfer, and the presence of specialized shunts that bypass the non-functional fetal lungs and partially functional liver. The umbilical cord, containing two arteries and one vein, serves as the vital conduit. The umbilical vein delivers highly oxygenated blood from the placenta to the fetus. Key fetal shunts include the ductus venosus, which bypasses the liver and shunts blood to the inferior vena cava; the foramen ovale, which allows blood to shunt from the right atrium to the left atrium, bypassing the pulmonary circulation; and the ductus arteriosus, which connects the pulmonary artery to the aorta, diverting blood away from the lungs. Fetal hemoglobin (HbF) possesses a higher affinity for oxygen, optimizing oxygen uptake from the maternal circulation.
Monitoring fetal heart rate (FHR) is paramount for assessing fetal well-being. A normal baseline FHR ranges from 110-160 bpm, with deviations indicating tachycardia (e.g., maternal fever, fetal hypoxia) or bradycardia (e.g., maternal hypotension, severe hypoxia). FHR variability reflects autonomic nervous system integrity, with moderate variability being the most reassuring sign. Accelerations are transient increases in FHR, indicative of fetal health. Decelerations categorize into early (benign, due to head compression), late (concerning, due to uteroplacental insufficiency), and variable (due to cord compression), with prolonged decelerations demanding immediate attention.
Fetal well-being assessments like the Non-Stress Test (NST) evaluate FHR accelerations, while the Biophysical Profile (BPP) combines NST with ultrasound parameters (breathing movements, gross body movements, tone, amniotic fluid volume) for a comprehensive assessment of fetal status.
The transition from fetal to neonatal circulation at birth involves dramatic physiological changes. Lung expansion and increased oxygenation lead to a sharp decrease in pulmonary vascular resistance (PVR). Simultaneously, clamping of the umbilical cord results in a significant increase in systemic vascular resistance (SVR). These pressure shifts trigger the functional closure of the fetal shunts: the ductus venosus becomes the ligamentum venosum, the foramen ovale becomes the fossa ovalis, and the ductus arteriosus becomes the ligamentum arteriosum. The umbilical arteries become medial umbilical ligaments, and the umbilical vein becomes the ligamentum teres. Understanding these intricate adaptations is crucial for nurses to provide optimal care to both mother and newborn, identifying and managing potential complications during this critical transitional period.
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Fetal Circulation
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


