Please set your exam date
Hypertensive Disorders
Study Questions
Practice Exercise 1
Which of the following defines gestational hypertension?
Explanation
Gestational hypertension is a pregnancy-specific hypertensive disorder characterized by new-onset elevated blood pressure after mid-gestation without accompanying proteinuria or systemic features. It typically arises after 20 weeks of gestation and resolves postpartum. The diagnostic threshold is systolic ≥140 mmHg or diastolic ≥90 mmHg on two occasions at least 4 hours apart. It must be distinguished from preeclampsia, which includes proteinuria or end-organ dysfunction, and from chronic hypertension, which occurs before 20 weeks or persists beyond 12 weeks postpartum.
Rationale for correct answer
3. Blood pressure ≥140/90 mmHg after 20 weeks without proteinuria defines gestational hypertension. The absence of proteinuria or systemic signs differentiates it from preeclampsia. The timing after 20 weeks is critical for diagnosis, and the threshold values match the accepted criteria.
Rationale for incorrect answers
1. Blood pressure ≥140/90 mmHg before 20 weeks indicates chronic hypertension, not gestational hypertension. Hypertension diagnosed before 20 weeks is presumed to be pre-existing and is not pregnancy-induced. The timing here excludes gestational hypertension.
2. Blood pressure ≥140/90 mmHg after 20 weeks with proteinuria defines preeclampsia, not gestational hypertension. The presence of proteinuria (≥300 mg in 24 hours or protein/creatinine ratio ≥0.3) indicates renal involvement and systemic disease, which is a hallmark of preeclampsia.
4. Blood pressure ≥160/110 mmHg with seizures defines eclampsia, a severe complication of preeclampsia. Seizures in a hypertensive pregnant woman indicate neurological involvement and progression beyond gestational hypertension. This is a medical emergency requiring immediate intervention.
Take home points
- Gestational hypertension is defined by new-onset hypertension after 20 weeks without proteinuria.
- Preeclampsia includes hypertension with proteinuria or systemic features.
- Chronic hypertension is diagnosed before 20 weeks or persists postpartum.
- Eclampsia involves seizures and is a complication of preeclampsia.
What is a key clinical manifestation of preeclampsia without severe features?
Explanation
Preeclampsia without severe features is a hypertensive disorder of pregnancy characterized by new-onset hypertension and proteinuria after 20 weeks of gestation. It involves systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg on two occasions at least 4 hours apart, plus proteinuria ≥300 mg in 24 hours, or protein/creatinine ratio ≥0.3. It lacks severe features such as thrombocytopenia, elevated liver enzymes, renal insufficiency, pulmonary edema, or cerebral/visual symptoms. The condition arises from abnormal placentation leading to systemic endothelial dysfunction and increased vascular permeability.
Rationale for correct answer
2. Proteinuria ≥300 mg in 24 hours is a defining feature of preeclampsia without severe features. It reflects glomerular endothelial injury and increased permeability. The absence of other systemic signs confirms the classification as “without severe features.”
Rationale for incorrect answers
1. Platelet count <100,000/μL indicates thrombocytopenia, a severe feature of preeclampsia. It reflects platelet consumption due to endothelial activation and microvascular injury. This finding upgrades the diagnosis to preeclampsia with severe features.
3. Blood pressure ≥160/110 mmHg meets the threshold for severe hypertension, which is a severe feature of preeclampsia. It increases risk for stroke and other complications, requiring urgent antihypertensive therapy and closer monitoring.
4. Pulmonary edema is a life-threatening complication and a severe feature of preeclampsia. It results from capillary leak and reduced oncotic pressure, often requiring intensive care and delivery planning. Its presence excludes the “without severe features” classification.
Take home points
- Preeclampsia without severe features includes hypertension and proteinuria after 20 weeks.
- Severe features include thrombocytopenia, elevated liver enzymes, renal dysfunction, and pulmonary edema.
- Blood pressure ≥160/110 mmHg is a severe feature.
- Proteinuria ≥300 mg in 24 hours is sufficient for diagnosis without severe features.
A 28-year-old primigravida at 34 weeks gestation presents with new-onset blood pressure readings of 150/98 mmHg and 148/96 mmHg four hours apart, along with 2+ proteinuria on a dipstick. She denies headache or visual changes. Which of the following is the most likely diagnosis?
Explanation
Preeclampsia without severe features is a pregnancy-specific hypertensive disorder that arises after 20 weeks gestation and is characterized by new-onset hypertension and proteinuria without evidence of end-organ dysfunction. Diagnostic criteria include systolic blood pressure ≥140 mmHg or diastolic ≥90 mmHg on two occasions at least 4 hours apart, plus proteinuria ≥300 mg/24 hours, protein/creatinine ratio ≥0.3, or dipstick ≥1+. It results from abnormal placentation leading to systemic endothelial dysfunction, vasospasm, and increased vascular permeability. Severe features include thrombocytopenia (<100,000/μL), elevated liver enzymes, renal insufficiency (creatinine >1.1 mg/dL), pulmonary edema, or cerebral/visual symptoms.
Rationale for correct answer
3. The patient has blood pressure readings ≥140/90 mmHg on two occasions 4 hours apart and 2+ proteinuria on dipstick, meeting the criteria for preeclampsia without severe features. The absence of neurologic symptoms or other systemic signs confirms the lack of severe features.
Rationale for incorrect answers
1. Gestational hypertension is defined as new-onset hypertension after 20 weeks without proteinuria or systemic signs. This patient has 2+ proteinuria, which excludes gestational hypertension and supports a diagnosis of preeclampsia.
2. Chronic hypertension is diagnosed when elevated blood pressure is present before 20 weeks gestation or persists beyond 12 weeks postpartum. This patient is 34 weeks gestation with new-onset hypertension, making chronic hypertension unlikely.
4. Preeclampsia with severe features requires signs of end-organ dysfunction, such as severe hypertension (≥160/110 mmHg), thrombocytopenia, elevated liver enzymes, renal insufficiency, pulmonary edema, or CNS symptoms. This patient lacks all severe features, making this diagnosis incorrect.
Take home points
- Preeclampsia without severe features includes hypertension and proteinuria after 20 weeks without systemic involvement.
- Severe features include thrombocytopenia, elevated liver enzymes, renal dysfunction, pulmonary edema, or CNS symptoms.
- Gestational hypertension lacks proteinuria and systemic signs.
Which of the following are risk factors for developing preeclampsia? Select all that apply
Explanation
Preeclampsia is a multisystem disorder of pregnancy characterized by new-onset hypertension and proteinuria or systemic involvement after 20 weeks gestation. It results from abnormal placentation, leading to widespread endothelial dysfunction, vasospasm, and increased vascular permeability. Risk factors include nulliparity, chronic hypertension, obesity, advanced maternal age, diabetes, renal disease, and multiple gestation. The condition typically presents after 20 weeks and may progress to severe features or eclampsia if untreated.
Rationale for correct answers
1. Nulliparity is a well-established risk factor due to lack of prior maternal immune adaptation to fetal antigens. Immune maladaptation and vascular remodeling defects are more common in first pregnancies, increasing susceptibility to preeclampsia.
2. Chronic hypertension predisposes to preeclampsia due to pre-existing vascular damage and endothelial dysfunction. These changes amplify the placental ischemia and systemic inflammation seen in preeclampsia.
4. Obesity increases risk through chronic inflammation and insulin resistance, which contribute to endothelial dysfunction. Adipose tissue also alters cytokine profiles and promotes hypertension, enhancing susceptibility.
Rationale for incorrect answers
3. Gestational age <20 weeks is not a risk factor but rather excludes the diagnosis of preeclampsia. The condition cannot occur before 20 weeks because placental development and vascular remodeling are incomplete. Hypertension before 20 weeks suggests chronic hypertension or other etiologies.
5. Previous cesarean delivery is not a recognized independent risk factor for preeclampsia. While it may be associated with placental abnormalities in future pregnancies, such as accreta or previa, it does not directly increase the risk of endothelial dysfunction or abnormal placentation.
Take home points
- Nulliparity, chronic hypertension, and obesity are major risk factors for preeclampsia.
- Preeclampsia occurs after 20 weeks gestation and involves systemic endothelial dysfunction.
- Chronic inflammation and immune maladaptation contribute to pathogenesis.
- Previous cesarean delivery is not a direct risk factor for preeclampsia.
Which of the following are recognized risk factors for developing preeclampsia? Select all that apply
Explanation
Preeclampsia is a hypertensive disorder of pregnancy marked by new-onset hypertension and proteinuria or systemic involvement after 20 weeks gestation. It arises from abnormal placental development, leading to endothelial dysfunction, vasospasm, and increased vascular permeability. Risk factors include primigravidity, chronic hypertension, multifetal gestation, prior gestational hypertension, diabetes, renal disease, and advanced maternal age. The condition may progress to severe features or eclampsia if not managed appropriately.
Rationale for correct answers
1. Primigravidity is a major risk factor due to lack of prior maternal immune adaptation to fetal antigens. Immune maladaptation and defective spiral artery remodeling increase susceptibility to placental ischemia and systemic inflammation.
2. History of gestational hypertension in a prior pregnancy increases recurrence risk due to persistent vascular sensitivity and placental dysfunction. Prior endothelial injury may predispose to future hypertensive disorders.
3. Chronic hypertension contributes through pre-existing vascular damage and impaired endothelial regulation, which amplify the pathophysiologic mechanisms of preeclampsia.
4. Multifetal gestation increases placental mass and angiogenic demand, heightening the risk of placental hypoperfusion and systemic endothelial activation.
Rationale for incorrect answer
5. Maternal age under 20 years is not a recognized independent risk factor. While younger age may be associated with socioeconomic disparities or limited prenatal care, it does not directly contribute to the pathophysiology of preeclampsia. Advanced maternal age (>35 years) is a more established risk factor due to vascular aging and comorbidities.
Take home points
- Primigravidity, chronic hypertension, and multifetal gestation are strong risk factors for preeclampsia.
- Prior gestational hypertension increases recurrence risk.
- Preeclampsia arises from abnormal placentation and systemic endothelial dysfunction.
- Maternal age under 20 years is not a direct risk factor.
Practice Exercise 2
A nurse is reviewing the pathophysiology of preeclampsia with a group of student nurses. Which of the following best describes the primary underlying cause of the disorder?
Explanation
Preeclampsia is a pregnancy-specific hypertensive disorder characterized by abnormal placental development, endothelial dysfunction, vasoconstriction, and systemic inflammation. The central pathology involves inadequate remodeling of spiral arteries by cytotrophoblasts, leading to high-resistance uteroplacental circulation. This results in placental hypoxia, oxidative stress, and release of antiangiogenic factors like sFlt-1 and endoglin. Clinical features include systolic blood pressure ≥140 mmHg, diastolic ≥90 mmHg, proteinuria ≥300 mg/24 hr, and elevated liver enzymes. Severe cases may show thrombocytopenia, pulmonary edema, or cerebral symptoms. Risk factors include nulliparity, chronic hypertension, diabetes, and autoimmune disorders.
Rationale for correct answer
2. Incomplete trophoblastic invasion of spiral arteries is the primary defect in preeclampsia. Normally, cytotrophoblasts invade maternal spiral arteries, transforming them into low-resistance vessels. In preeclampsia, this invasion is shallow and incomplete, resulting in placental ischemia and hypoxia. This triggers systemic endothelial dysfunction and hypertension.
Rationale for incorrect answers
1. Excessive salt intake does not cause preeclampsia. Although salt can influence blood pressure, preeclampsia is not a salt-sensitive condition. The disorder arises from placental malperfusion, not dietary sodium. Studies show no consistent link between sodium intake and preeclampsia incidence.
3. Increased cardiac output and plasma volume are normal physiological adaptations in pregnancy. These changes support fetal growth and do not cause preeclampsia. In fact, preeclampsia often presents with reduced plasma volume and vasoconstriction, not hypervolemia. The pathology is vascular, not volume-driven.
4. Overproduction of placental prostacyclin is not implicated in preeclampsia. Prostacyclin is a vasodilator and platelet inhibitor. In preeclampsia, there is a relative deficiency of prostacyclin and excess thromboxane A2, leading to vasoconstriction and platelet aggregation. The imbalance favors hypertension and endothelial damage.
Take home points
- Preeclampsia arises from defective spiral artery remodeling by trophoblasts.
- Placental ischemia leads to systemic endothelial dysfunction and hypertension.
- Normal pregnancy adaptations like increased plasma volume do not cause preeclampsia.
- Preeclampsia involves reduced prostacyclin and increased thromboxane A2.
The nurse understands that the endothelial dysfunction in preeclampsia leads to which of the following physiologic changes?
Explanation
Endothelial dysfunction in preeclampsia is a central pathological feature resulting from placental ischemia, oxidative stress, antiangiogenic factor release, and immune activation. The dysfunctional endothelium loses its ability to regulate vascular tone, permeability, coagulation, and inflammatory responses. This leads to systemic vasoconstriction, increased vascular permeability, and activation of the coagulation cascade. Nitric oxide production is reduced, while sensitivity to vasoconstrictors like angiotensin II and endothelin-1 is heightened. The imbalance between prostacyclin and thromboxane A2 favors vasoconstriction and platelet aggregation. Clinical consequences include hypertension, proteinuria, edema, and organ dysfunction.
Rationale for correct answer
3. Generalized vasoconstriction and capillary leak are hallmark features of endothelial dysfunction in preeclampsia. The endothelium loses its barrier integrity, allowing plasma proteins to leak into interstitial spaces, causing edema. Simultaneously, reduced nitric oxide and increased vasoconstrictors lead to hypertension and organ hypoperfusion.
Rationale for incorrect answers
1. Increased production of nitric oxide does not occur in preeclampsia. In fact, nitric oxide synthesis is reduced due to endothelial damage. This contributes to vasoconstriction and impaired uteroplacental perfusion. The imbalance favors vasoconstrictors like endothelin-1 and thromboxane A2.
2. Decreased vascular sensitivity to angiotensin II is incorrect. In preeclampsia, there is increased sensitivity to angiotensin II, contributing to hypertension. The vasculature responds excessively to circulating vasoconstrictors due to endothelial dysfunction and reduced nitric oxide.
4. Reduced platelet activation and aggregation is inaccurate. Preeclampsia is associated with increased platelet activation, leading to microthrombi formation and consumption of platelets. This contributes to thrombocytopenia and disseminated intravascular coagulation in severe cases.
Take home points
- Endothelial dysfunction in preeclampsia causes vasoconstriction and capillary leak.
- Nitric oxide production is reduced, enhancing vascular tone.
- Angiotensin II sensitivity is increased, not decreased.
A client with severe preeclampsia presents with oliguria, elevated creatinine, and proteinuria. The nurse recognizes that these findings primarily result from which of the following?
Explanation
Renal involvement in severe preeclampsia is driven by glomerular endotheliosis, renal vasospasm, endothelial dysfunction, and reduced perfusion. The hallmark lesion is glomerular endotheliosis, characterized by swelling of glomerular endothelial cells, narrowing of capillary lumens, and loss of fenestrations. This leads to decreased glomerular filtration rate (GFR), oliguria, and elevated serum creatinine. Proteinuria results from increased glomerular permeability due to endothelial injury. Renal vasospasm further reduces renal blood flow, exacerbating ischemia and tubular dysfunction. These changes are reversible postpartum but may progress to acute kidney injury in severe cases.
Rationale for correct answer
2. Glomerular endotheliosis and renal vasospasm are the primary renal lesions in preeclampsia. Endothelial swelling narrows glomerular capillaries, reducing filtration surface and GFR. Vasospasm decreases renal perfusion, worsening ischemia and oliguria. Proteinuria arises from increased glomerular permeability due to endothelial damage.
Rationale for incorrect answers
1. Hepatic microvascular thrombosis is associated with HELLP syndrome, not the renal findings described. It causes elevated liver enzymes and right upper quadrant pain, not oliguria or creatinine rise. The renal pathology in preeclampsia is glomerular, not hepatic.
3. Increased glomerular filtration rate is a normal pregnancy adaptation, not a feature of preeclampsia. In preeclampsia, GFR is reduced due to glomerular endotheliosis and vasoconstriction. Elevated creatinine and oliguria reflect decreased filtration, not enhancement.
4. Immune complex deposition is characteristic of glomerulonephritis, not preeclampsia. Preeclampsia lacks immune-mediated inflammation or complement activation. The renal lesion is non-inflammatory, with endothelial swelling and capillary narrowing.
Take home points
- Glomerular endotheliosis is the hallmark renal lesion in preeclampsia.
- Renal vasospasm reduces perfusion, causing oliguria and elevated creatinine.
- Proteinuria results from endothelial injury and increased glomerular permeability.
- Immune complex deposition is absent in preeclampsia; it suggests glomerulonephritis.
A nurse caring for a woman with preeclampsia notes platelet count of 85,000/μL and elevated liver enzymes. Which pathophysiologic mechanism explains these findings?
Explanation
HELLP syndrome is a severe variant of preeclampsia characterized by Hemolysis, Elevated Liver enzymes, and Low Platelets. It results from widespread endothelial dysfunction, microvascular injury, and platelet activation. The hepatic sinusoids and systemic vasculature develop fibrin-rich thrombi, causing red blood cell fragmentation and hepatocellular injury. Platelet consumption leads to thrombocytopenia, and liver enzyme elevation reflects hepatocyte necrosis. Hemolysis is microangiopathic, not immune-mediated. HELLP typically presents in the third trimester with right upper quadrant pain, nausea, and malaise. Platelet count <100,000/μL and AST or ALT >70 IU/L are diagnostic thresholds.
Rationale for correct answer
2. Platelet aggregation and microangiopathic hemolysis explain the findings. Endothelial injury triggers platelet activation, forming microthrombi in hepatic and systemic vessels. Red blood cells are sheared as they pass through narrowed capillaries, causing hemolysis. Platelet consumption leads to thrombocytopenia, and hepatocyte ischemia elevates liver enzymes.
Rationale for incorrect answers
1. Excessive prostacyclin production and vasodilation are not features of HELLP syndrome. In preeclampsia and HELLP, prostacyclin is reduced, favoring vasoconstriction and platelet aggregation. Vasodilation would oppose thrombosis and organ ischemia, which are central to HELLP pathology.
3. Autoimmune destruction of red blood cells is characteristic of autoimmune hemolytic anemia, not HELLP. HELLP involves mechanical fragmentation of red cells due to microvascular thrombi, not antibody-mediated lysis. Coombs test is negative in HELLP.
4. Decreased fibrin deposition is incorrect. HELLP involves increased fibrin deposition in microvasculature, contributing to thrombotic microangiopathy. This leads to platelet consumption, hemolysis, and organ damage. Reduced fibrin would not explain thrombocytopenia or liver injury.
Take home points
- HELLP syndrome involves hemolysis, elevated liver enzymes, and low platelets.
- Microangiopathic hemolysis results from red cell fragmentation in fibrin-rich vessels.
- Platelet activation and consumption cause thrombocytopenia.
The nurse understands that generalized vasospasm in preeclampsia causes which of the following clinical manifestations?
Explanation
Generalized vasospasm in preeclampsia results from endothelial dysfunction, reduced nitric oxide, increased vasoconstrictors, and placental ischemia. The systemic vasoconstriction affects multiple organs, leading to hypertension, organ hypoperfusion, and capillary leak. Renal vasospasm reduces glomerular filtration, causing oliguria and proteinuria. Cerebral vasospasm leads to visual disturbances and risk of seizures. Hepatic ischemia causes epigastric pain and elevated liver enzymes. Vasospasm also contributes to placental insufficiency and fetal growth restriction. Blood pressure is elevated, not decreased, due to systemic vascular resistance.
Rationale for correct answers
1. Blurred vision results from cerebral vasospasm and retinal edema. Vasoconstriction of cerebral vessels impairs perfusion, causing visual disturbances such as scotomata, photopsia, and diplopia. Severe cases may progress to cortical blindness or eclampsia.
2. Decreased urine output is due to renal vasospasm and glomerular endotheliosis. Vasoconstriction reduces renal perfusion and glomerular filtration rate, leading to oliguria. This reflects worsening renal involvement and risk of acute kidney injury.
4. Epigastric pain arises from hepatic ischemia and capsular distension. Vasospasm in hepatic sinusoids causes hepatocyte injury and elevated liver enzymes. The pain is typically right upper quadrant and may signal impending HELLP syndrome.
5. Proteinuria results from glomerular endothelial injury and increased permeability. Vasospasm and endotheliosis disrupt the filtration barrier, allowing albumin and other proteins to leak into urine. Proteinuria ≥300 mg/24 hr is diagnostic.
Rationale for incorrect answer
3. Decreased blood pressure is incorrect. Preeclampsia is defined by elevated blood pressure due to systemic vasoconstriction. Systolic ≥140 mmHg or diastolic ≥90 mmHg after 20 weeks gestation is diagnostic. Hypotension is not a feature unless there is hemorrhage or shock.
Take home points
- Vasospasm in preeclampsia causes organ hypoperfusion and systemic hypertension.
- Cerebral vasospasm leads to visual symptoms and seizure risk.
- Renal vasospasm causes oliguria and proteinuria.
- Hepatic ischemia presents as epigastric pain and elevated liver enzymes.
Practice Exercise 3
A 28-year-old primigravida at 34 weeks gestation presents with new-onset BP 150/98 mmHg and 2+ proteinuria. Which of the following is the most likely diagnosis?
Explanation
Preeclampsia is a multisystem disorder of pregnancy characterized by new-onset hypertension, proteinuria, and endothelial dysfunction after 20 weeks gestation. It results from abnormal placentation leading to systemic vasoconstriction and increased vascular permeability. Diagnostic criteria include systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg on two occasions at least 4 hours apart, and proteinuria ≥300 mg/24 hours or ≥1+ on dipstick. Severe features include BP ≥160/110 mmHg, thrombocytopenia <100,000/µL, elevated liver enzymes, renal insufficiency (creatinine >1.1 mg/dL), pulmonary edema, or cerebral/visual symptoms.
Rationale for correct answer
3. The patient is at 34 weeks gestation with new-onset BP of 150/98 mmHg and 2+ proteinuria, meeting criteria for preeclampsia. There are no signs of severe features such as BP ≥160/110 mmHg, thrombocytopenia, elevated creatinine, or neurological symptoms. The absence of these severe features and the presence of proteinuria confirm preeclampsia without severe features.
Rationale for incorrect answers
1. Gestational hypertension is defined as new-onset hypertension after 20 weeks gestation without proteinuria or systemic signs. This patient has 2+ proteinuria, which excludes gestational hypertension. The presence of proteinuria and systemic involvement rules out this diagnosis.
2. Chronic hypertension is diagnosed when elevated BP is present before pregnancy or before 20 weeks gestation. This patient is a primigravida at 34 weeks with new-onset hypertension, making chronic hypertension unlikely. The timing and absence of prior history exclude chronic hypertension.
4. Preeclampsia with severe features requires either BP ≥160/110 mmHg or evidence of end-organ damage. This patient’s BP is 150/98 mmHg and there are no signs of thrombocytopenia, renal dysfunction, or neurological symptoms. The lack of end-organ damage and severe hypertension makes this diagnosis incorrect.
Take home points
- Preeclampsia is diagnosed after 20 weeks gestation with hypertension and proteinuria.
- Severe features include BP ≥160/110 mmHg or signs of end-organ damage.
- Gestational hypertension lacks proteinuria and systemic involvement.
- Chronic hypertension is diagnosed before 20 weeks gestation or pre-pregnancy.
Which laboratory finding is indicative of hemolysis in HELLP syndrome?
Explanation
HELLP syndrome is a severe variant of preeclampsia characterized by hemolysis, elevated liver enzymes, and low platelets. It typically presents in the third trimester and may progress rapidly. Hemolysis results from microangiopathic destruction of red blood cells due to endothelial injury and fibrin deposition in small vessels. Diagnostic markers include elevated lactate dehydrogenase (>600 IU/L), low haptoglobin, and presence of schistocytes on peripheral smear. Liver enzymes (AST, ALT) are elevated, and platelet count is typically <100,000/µL. HELLP may present with right upper quadrant pain, nausea, and hypertension.
Rationale for correct answer
3. Schistocytes are fragmented red blood cells seen in microangiopathic hemolytic anemia, a hallmark of HELLP syndrome. Their presence on peripheral smear confirms intravascular hemolysis due to endothelial damage, making this the most specific laboratory finding.
Rationale for incorrect answers
1. Elevated platelet count is not consistent with HELLP syndrome. Thrombocytopenia (<100,000/µL) is a defining feature due to platelet consumption and vascular injury. An elevated count would argue against the diagnosis.
2. Decreased lactate dehydrogenase is incorrect. LDH is released during cell lysis and is typically elevated (>600 IU/L) in HELLP due to hemolysis and hepatic injury. A decreased level would not support the diagnosis.
4. Normal haptoglobin levels are not indicative of hemolysis. Haptoglobin binds free hemoglobin and is consumed during hemolysis, leading to low levels. Normal levels suggest absence of significant red cell destruction.
Take home points
- HELLP syndrome involves hemolysis, elevated liver enzymes, and low platelets.
- Schistocytes confirm microangiopathic hemolysis.
- LDH is elevated due to cell lysis; haptoglobin is decreased.
- Platelet count is reduced due to consumption in vascular injury.
Which of the following findings would indicate that preeclampsia has severe features? Select all that apply
Explanation
Preeclampsia with severe features is a hypertensive disorder of pregnancy marked by end-organ dysfunction, vascular injury, and systemic inflammation. It is diagnosed when preeclampsia is accompanied by one or more severe features: BP ≥160/110 mmHg, platelet count <100,000/μL, serum creatinine >1.1 mg/dL, elevated liver enzymes, pulmonary edema, or neurological symptoms such as visual disturbances or altered mental status. Oliguria (<500 mL/24h) and signs of cerebral involvement also indicate severity. These features reflect widespread endothelial damage and microvascular compromise.
Rationale for correct answers
1. A platelet count of 90,000/μL meets the criterion for thrombocytopenia (<100,000/μL), indicating vascular injury and platelet consumption, consistent with severe features.
2. Serum creatinine of 1.5 mg/dL exceeds the threshold of >1.1 mg/dL or doubling of baseline, indicating renal impairment and end-organ dysfunction, a hallmark of severe preeclampsia.
3. Temporary blindness reflects cerebral involvement and neurological dysfunction, which are considered severe features due to risk of eclampsia and stroke.
5. Urine output of 700 mL/24h is reduced but not below the strict oliguria threshold (<500 mL/24h). However, it suggests renal hypoperfusion and progressive dysfunction, which may be considered a severe feature in clinical context.
Rationale for incorrect answer
4. BP of 145/95 mmHg does not meet the threshold for severe hypertension (≥160/110 mmHg). While elevated, it reflects moderate hypertension and lacks the pressure level required to classify as severe. Without accompanying end-organ signs, this BP alone is insufficient.
Take home points
- Severe features of preeclampsia include thrombocytopenia, renal dysfunction, and neurological symptoms.
- BP ≥160/110 mmHg is required to meet the severe hypertension criterion.
- Visual disturbances signal cerebral involvement and risk of eclampsia.
- Oliguria and elevated creatinine reflect renal compromise.
Which fetal complication is associated with hypertensive disorders in pregnancy?
Explanation
Hypertensive disorders in pregnancy such as preeclampsia and gestational hypertension impair uteroplacental perfusion, leading to fetal hypoxia, nutrient deprivation, and growth restriction. The underlying pathology involves vasospasm, endothelial dysfunction, and placental ischemia, which reduce oxygen and nutrient delivery to the fetus. This results in intrauterine growth restriction (IUGR), low birth weight, and increased risk of preterm birth. Doppler studies often show abnormal umbilical artery flow. These pregnancies require close monitoring of fetal growth and amniotic fluid volume.
Rationale for correct answer
2. Intrauterine growth restriction occurs due to impaired placental perfusion and chronic fetal hypoxia caused by maternal hypertension. The fetus receives inadequate nutrients and oxygen, leading to reduced growth velocity and low estimated fetal weight.
Rationale for incorrect answers
1. Macrosomia is typically associated with maternal diabetes, not hypertension. It results from hyperglycemia-induced fetal hyperinsulinemia, promoting excessive growth. Hypertensive pregnancies more commonly lead to restricted growth, not overgrowth.
3. Polyhydramnios is linked to fetal anomalies (e.g., anencephaly), maternal diabetes, or idiopathic causes. Hypertensive disorders often cause oligohydramnios due to reduced placental function and fetal urine output, not excess fluid.
4. Congenital heart defects are not directly caused by maternal hypertension. They are more often associated with genetic syndromes or teratogenic exposures during organogenesis. Hypertension affects placental function but not structural fetal development.
Take home points
- Hypertensive disorders impair placental perfusion, leading to fetal growth restriction.
- Macrosomia is linked to maternal diabetes, not hypertension.
- Polyhydramnios is not typical in hypertensive pregnancies; oligohydramnios is more common.
- Congenital anomalies are not directly caused by maternal hypertension.
A nurse is caring for a client with HELLP syndrome. Which laboratory findings are expected? Select all that apply
Explanation
HELLP syndrome is a severe form of preeclampsia characterized by hemolysis, elevated liver enzymes, and low platelets. It results from widespread endothelial dysfunction, leading to microangiopathic hemolytic anemia, hepatic injury, and thrombocytopenia. Hemolysis causes elevated lactate dehydrogenase (>600 IU/L), decreased haptoglobin, and presence of schistocytes. Liver damage elevates AST and ALT, often >70 IU/L. Platelet count typically drops below 100,000/μL due to consumption. Bilirubin may be elevated due to hemolysis, not decreased.
Rationale for correct answers
1. Elevated LDH reflects cellular breakdown and hemolysis, a hallmark of HELLP. LDH >600 IU/L is a diagnostic marker due to red cell destruction and hepatic injury.
2. Decreased platelet count (<100,000/μL) is due to platelet consumption and vascular damage, fulfilling the “LP” component of HELLP.
3. Elevated AST/ALT indicates hepatic injury from microvascular damage and fibrin deposition. Levels often exceed 70 IU/L, confirming liver involvement.
Rationale for incorrect answers
4. Elevated haptoglobin is not expected. Haptoglobin binds free hemoglobin and is consumed during hemolysis, leading to low levels. Elevated values suggest absence of red cell destruction.
5. Decreased bilirubin is incorrect. Hemolysis increases unconjugated bilirubin, so levels are typically elevated. A decrease would not reflect hemolytic activity.
Take home points
- HELLP syndrome involves hemolysis, elevated liver enzymes, and low platelets.
- LDH and AST/ALT are elevated due to cellular injury.
- Haptoglobin is decreased in hemolysis.
- Bilirubin rises due to red cell breakdown.
Practice Exercise 4
A nurse is monitoring a client with severe preeclampsia receiving magnesium sulfate. Which assessment finding requires immediate intervention?
Explanation
Magnesium sulfate toxicity is a serious complication in obstetric care, especially in clients with severe preeclampsia. Magnesium sulfate is used to prevent seizures by depressing neuromuscular transmission and central nervous system activity. Toxicity manifests with hyporeflexia, respiratory depression, and cardiac arrest. Therapeutic serum magnesium levels range from 4.8 to 8.4 mg/dL. Toxicity risk increases with renal impairment, as magnesium is excreted renally. Early signs include loss of deep tendon reflexes and respiratory rate below 12 breaths per minute. Late signs include hypotension and bradycardia.
Rationale for correct answer
1. A respiratory rate of 10 breaths per minute indicates respiratory depression, a hallmark of magnesium sulfate toxicity. Magnesium inhibits acetylcholine release at neuromuscular junctions, leading to muscle weakness and decreased respiratory drive. Immediate intervention is required to prevent progression to apnea and cardiac arrest.
Rationale for incorrect answers
2. The presence of 2+ deep tendon reflexes is a normal finding and suggests that magnesium levels are within the therapeutic range. Loss of reflexes is an early sign of toxicity, so preserved reflexes indicate no immediate concern. Reflexes are monitored to assess neuromuscular function during magnesium therapy.
3. Urine output of 40 mL/hour is above the minimum threshold of 30 mL/hour required for safe magnesium excretion. Although renal function must be monitored closely, this output does not indicate renal compromise or toxicity. Magnesium clearance depends on glomerular filtration, and this rate is adequate.
4. Mild flushing of the skin is a common and benign side effect of magnesium sulfate due to vasodilation. It does not indicate toxicity and typically resolves spontaneously. Flushing is related to peripheral vascular effects and does not require intervention.
Take home points
- Magnesium sulfate toxicity presents with respiratory depression, hyporeflexia, and cardiac arrest.
- Therapeutic magnesium levels range from 4.8 to 8.4 mg/dL.
- Deep tendon reflexes are the earliest clinical indicator of toxicity.
- Renal function must be monitored to prevent magnesium accumulation.
Which medication is commonly used for seizure prophylaxis in severe preeclampsia?
Explanation
Severe preeclampsia is a hypertensive disorder of pregnancy characterized by blood pressure ≥160/110 mmHg, proteinuria ≥0.3 g/24h, and signs of end-organ dysfunction. It poses a high risk for eclampsia, defined by new-onset seizures. The cornerstone of seizure prophylaxis is magnesium sulfate, which acts as a central nervous system depressant by blocking neuromuscular transmission and reducing cerebral vasospasm. It is not an antihypertensive but is essential for preventing convulsions. Therapeutic serum magnesium levels range from 4.8 to 8.4 mg/dL. Toxicity presents with hyporeflexia, respiratory depression, and cardiac arrest, especially in renal impairment.
Rationale for correct answer
3. Magnesium sulfate is the drug of choice for seizure prophylaxis in severe preeclampsia. It reduces the risk of progression to eclampsia by stabilizing neuronal membranes and decreasing excitability. It does not lower blood pressure but is critical in preventing maternal morbidity from seizures.
Rationale for incorrect answers
1. Labetalol is a beta-blocker used to manage acute hypertension in preeclampsia. It reduces systemic vascular resistance but has no anticonvulsant properties. It is not used for seizure prevention and does not affect neuronal excitability.
2. Hydralazine is a direct vasodilator used to lower diastolic blood pressure in hypertensive emergencies. It is effective for blood pressure control but lacks central nervous system depressant effects. It does not prevent seizures and is not the standard for eclampsia prophylaxis.
4. Nifedipine is a calcium channel blocker used for blood pressure reduction in pregnancy. It relaxes vascular smooth muscle but does not cross the blood-brain barrier to exert anticonvulsant effects. It is not indicated for seizure prevention in preeclampsia.
Take home points
- Magnesium sulfate is the first-line agent for seizure prophylaxis in severe preeclampsia.
- Antihypertensives like labetalol, hydralazine, and nifedipine manage blood pressure but do not prevent seizures.
- Eclampsia is defined by new-onset seizures in a preeclamptic patient.
- Magnesium toxicity presents with hyporeflexia and respiratory depression.
A patient receiving hydralazine IV for severe preeclampsia develops tachycardia and headache. What is the appropriate nursing action?
Explanation
Hydralazine adverse effects are common during intravenous administration, especially in obstetric patients with severe preeclampsia. Hydralazine is a direct arteriolar vasodilator used to lower diastolic blood pressure rapidly. It acts by relaxing vascular smooth muscle, leading to decreased systemic vascular resistance. However, this vasodilation can trigger reflex sympathetic activation, resulting in tachycardia, headache, and palpitations. These symptoms may indicate excessive hypotension or cerebral vasodilation, both of which are dangerous in preeclampsia. Hydralazine is contraindicated in patients with tachyarrhythmias, angina, or increased intracranial pressure.
Rationale for correct answer
1. Stopping the medication and notifying the provider is appropriate because tachycardia and headache suggest adverse effects from hydralazine-induced vasodilation. These symptoms may reflect excessive hypotension or cerebral hyperperfusion, both of which can worsen maternal and fetal outcomes. Immediate cessation prevents further hemodynamic instability.
Rationale for incorrect answers
2. Continuing the infusion and reassessing in 30 minutes is unsafe. Tachycardia and headache are not mild side effects but signs of hemodynamic stress. Delaying intervention risks progression to hypotension, fetal compromise, or stroke. Reassessment without action is inappropriate.
3. Administering another antihypertensive without evaluating the cause of symptoms may worsen the situation. Combining agents can lead to synergistic hypotension and further cardiac strain. The priority is to stop the offending drug and reassess before initiating new therapy.
4. Documenting and continuing observation ignores the clinical significance of the symptoms. Headache in preeclampsia may signal cerebral edema or vasospasm, and tachycardia may indicate compensatory response to hypotension. Passive observation delays necessary intervention.
Take home points
- Hydralazine causes reflex tachycardia and headache due to arteriolar vasodilation.
- These symptoms may indicate excessive hypotension or cerebral hyperperfusion.
- Immediate cessation and provider notification are required for adverse reactions.
- Monitoring for neurological and cardiovascular signs is critical in preeclampsia.
What is the antidote for magnesium sulfate toxicity?
Explanation
Magnesium sulfate toxicity is a life-threatening complication that can occur during treatment for severe preeclampsia or eclampsia. Magnesium sulfate depresses neuromuscular transmission and central nervous system activity, and toxicity manifests with hyporeflexia, respiratory depression, and cardiac arrest. The antidote is calcium gluconate, which antagonizes magnesium’s effects at the neuromuscular junction and restores muscle contractility. Calcium gluconate is administered IV, typically 1 g over 3 minutes. Magnesium is renally excreted, so toxicity risk increases with renal impairment. Therapeutic serum magnesium levels range from 4.8 to 8.4 mg/dL.
Rationale for correct answer
2. Calcium gluconate is the antidote for magnesium sulfate toxicity. It restores neuromuscular function by competing with magnesium at calcium channels and reversing respiratory depression. It is administered IV and acts rapidly to prevent progression to cardiac arrest.
Rationale for incorrect answers
1. Sodium bicarbonate is used to correct metabolic acidosis, not magnesium toxicity. It does not antagonize magnesium at the neuromuscular junction and has no role in reversing respiratory depression caused by magnesium.
3. Potassium chloride is used to treat hypokalemia, not magnesium toxicity. It can worsen cardiac conduction abnormalities if given during magnesium-induced bradycardia or heart block. It does not reverse magnesium’s neuromuscular effects.
4. Furosemide is a loop diuretic used to promote renal excretion of magnesium, but it is not an antidote. It acts slowly and is not suitable for acute reversal of toxicity. It may be used adjunctively after stabilization.
Take home points
- Calcium gluconate is the antidote for magnesium sulfate toxicity.
- Magnesium toxicity presents with hyporeflexia, respiratory depression, and cardiac arrest.
- Renal impairment increases the risk of magnesium accumulation.
Furosemide may aid magnesium excretion but is not an emergency antidote.
Which of the following are signs of magnesium sulfate toxicity? Select all that apply
Explanation
Magnesium sulfate toxicity occurs when serum magnesium levels exceed the therapeutic range of 4.8–8.4 mg/dL, leading to progressive neuromuscular and respiratory depression. Magnesium acts as a calcium antagonist at neuromuscular junctions, reducing acetylcholine release and impairing muscle contraction. Early signs include loss of deep tendon reflexes, followed by respiratory depression (rate <12 breaths/min), and eventually cardiac arrest. Magnesium is excreted renally, so decreased urine output increases the risk of accumulation. Monitoring reflexes, respiratory rate, and urine output is essential to detect toxicity early.
Rationale for correct answers
1. Loss of deep tendon reflexes is an early and reliable indicator of neuromuscular blockade due to magnesium’s inhibitory effect on acetylcholine release. It precedes respiratory depression and signals rising serum magnesium levels.
2. Respiratory rate <12 breaths/min reflects respiratory muscle paralysis from excessive magnesium. Magnesium depresses the medullary respiratory center, leading to hypoventilation and potential apnea.
3. Decreased urine output (<30 mL/hour) impairs renal clearance of magnesium, increasing the risk of toxic accumulation. This is especially dangerous in preeclamptic patients with renal involvement.
Rationale for incorrect answers
4. Hyperreflexia is not a sign of magnesium toxicity. It is typically seen in preeclampsia before magnesium administration. Magnesium suppresses reflexes; thus, hyperreflexia suggests subtherapeutic levels, not toxicity.
5. Increased blood pressure is a hallmark of preeclampsia, not magnesium toxicity. Magnesium sulfate does not raise blood pressure; it may cause vasodilation and mild hypotension. Elevated pressure indicates disease severity, not drug toxicity.
Take home points
- Magnesium toxicity presents with hyporeflexia, respiratory depression, and reduced urine output.
- Reflex monitoring is essential for early detection of toxicity.
- Hyperreflexia suggests subtherapeutic magnesium levels.
- Magnesium is excreted renally; oliguria increases toxicity risk.
Practice Exercise 5
Which laboratory finding is indicative of hemolysis in HELLP syndrome?
Explanation
HELLP syndrome is a severe variant of preeclampsia characterized by hemolysis, elevated liver enzymes, and low platelets. Hemolysis results from microangiopathic destruction of red blood cells due to endothelial dysfunction and fibrin deposition. Schistocytes, elevated LDH, low haptoglobin, and indirect hyperbilirubinemia are specific markers. Platelet count is typically <100,000/mm³. Liver enzymes, especially AST and ALT, are elevated above 70 IU/L. HELLP may progress to DIC, hepatic rupture, or renal failure.
Rationale for correct answer
3. Schistocytes are fragmented red blood cells seen on peripheral smear, indicating microangiopathic hemolytic anemia. Their presence confirms intravascular destruction due to endothelial damage and fibrin strands in HELLP syndrome. This is a direct marker of hemolysis, making it the most specific laboratory finding among the options.
Rationale for incorrect answers
1. Elevated platelet count is not consistent with HELLP syndrome. Instead, thrombocytopenia is a hallmark feature, often <100,000/mm³, due to platelet consumption in microvascular injury. An elevated count suggests absence of platelet destruction, ruling out HELLP.
2. Decreased lactate dehydrogenase contradicts the pathophysiology. LDH is released during cellular breakdown, especially from red blood cells and hepatocytes. In HELLP, LDH is typically elevated >600 IU/L due to ongoing hemolysis and liver injury. A decrease would suggest no active hemolysis.
4. Normal haptoglobin levels are not expected in HELLP syndrome. Haptoglobin binds free hemoglobin released during intravascular hemolysis. In HELLP, levels are decreased due to consumption. Normal values (30–200 mg/dL) would argue against active hemolysis.
Take home points
- HELLP syndrome involves hemolysis, elevated liver enzymes, and low platelets.
- Schistocytes confirm microangiopathic hemolysis.
- LDH and bilirubin are elevated; haptoglobin is decreased.
- HELLP must be differentiated from TTP, HUS, and acute fatty liver of pregnancy.
A nurse is caring for a client diagnosed with HELLP syndrome. The client suddenly complains of severe right upper quadrant pain. The nurse suspects which potentially life-threatening complication?
Explanation
HELLP syndrome is a severe obstetric complication involving hemolysis, elevated liver enzymes, and low platelets. It results from systemic endothelial dysfunction and microvascular injury, leading to hepatic inflammation and fibrin deposition. Right upper quadrant pain, elevated AST/ALT, thrombocytopenia, and schistocytes are hallmark findings. Liver involvement may progress to hepatic hematoma or rupture, especially with platelet counts <50,000/mm³. This can cause hemorrhage, hypotension, and shock. HELLP typically occurs between 28–36 weeks gestation and requires urgent delivery.
Rationale for correct answer
3. Hepatic subcapsular hematoma or rupture is a known life-threatening complication of HELLP syndrome. Severe right upper quadrant pain reflects hepatic distension or bleeding. The liver capsule stretches due to hematoma formation, and rupture may lead to hemoperitoneum and hypovolemic shock. This is an obstetric emergency requiring immediate surgical and supportive intervention.
Rationale for incorrect answers
1. Pulmonary embolism presents with pleuritic chest pain, dyspnea, and tachypnea, not localized right upper quadrant pain. Although HELLP increases thrombotic risk, PE does not cause hepatic capsule pain or liver rupture. No respiratory symptoms are described, making this less likely.
2. Placental abruption causes vaginal bleeding, uterine tenderness, and fetal distress, not right upper quadrant pain. It results from premature placental separation, often with hypertension, but does not involve hepatic pathology. The pain described is not uterine or lower abdominal.
4. Deep vein thrombosis presents with unilateral leg swelling, pain, and erythema, not right upper quadrant pain. While HELLP increases clot risk, DVT does not cause hepatic symptoms or liver rupture. No limb findings are noted in the scenario.
Take home points
- HELLP syndrome may lead to hepatic hematoma or rupture, presenting with right upper quadrant pain.
- Liver rupture is a surgical emergency with high maternal mortality.
- HELLP must be differentiated from other causes of abdominal pain in pregnancy.
- Pulmonary embolism and DVT present with respiratory or limb symptoms, not hepatic pain.
Which of the following are components of HELLP syndrome? Select all that apply
Explanation
HELLP syndrome is a severe obstetric complication characterized by hemolysis, elevated liver enzymes, and low platelet count. It typically occurs in the third trimester and is considered a variant of preeclampsia. Microangiopathic hemolysis, hepatic inflammation, thrombocytopenia, and endothelial dysfunction are central features. Hemolysis is confirmed by schistocytes, elevated LDH >600 IU/L, and low haptoglobin <25 mg/dL. Liver enzymes, especially AST and ALT, rise above 70 IU/L due to hepatocellular injury. Platelet counts fall below 100,000/mm³ due to consumption. HELLP may progress to DIC, hepatic rupture, or renal failure.
Rationale for correct answers
1. Hemolysis is a core diagnostic criterion of HELLP syndrome. It results from microvascular injury and fibrin deposition causing red cell fragmentation. Laboratory markers include schistocytes on smear, elevated LDH, and decreased haptoglobin.
2. Elevated liver enzymes reflect hepatocellular damage due to periportal necrosis and microvascular thrombosis. AST and ALT levels typically exceed 70 IU/L. This hepatic involvement contributes to right upper quadrant pain and risk of subcapsular hematoma.
4. Low platelet count (<100,000/mm³) is a defining feature due to platelet consumption in damaged vasculature. Thrombocytopenia increases bleeding risk and may progress to DIC. It is essential for diagnosis and guides urgency of delivery.
Rationale for incorrect answers
3. Hypoglycemia is not a component of HELLP syndrome. Glucose metabolism is not directly affected. In contrast, hyperglycemia may occur in gestational diabetes. HELLP affects liver and coagulation, not insulin regulation or glucose levels.
5. Hypertension is common in preeclampsia but not a diagnostic criterion of HELLP syndrome. While many patients with HELLP have elevated blood pressure, normotensive cases exist. Diagnosis relies on hemolysis, liver enzymes, and platelets—not blood pressure.
Take home points
- HELLP syndrome includes hemolysis, elevated liver enzymes, and low platelets.
- Hypertension may coexist but is not required for diagnosis.
- Hypoglycemia is unrelated to HELLP syndrome pathophysiology.
- HELLP must be differentiated from acute fatty liver of pregnancy and TTP.
The definitive treatment for both preeclampsia and HELLP syndrome is:
Explanation
Preeclampsia and HELLP syndrome are hypertensive disorders of pregnancy driven by placental dysfunction, endothelial injury, and systemic inflammation. The placenta releases antiangiogenic factors like sFlt-1 and soluble endoglin, leading to vasoconstriction, coagulopathy, and organ damage. HELLP adds microangiopathic hemolysis, hepatic injury, and thrombocytopenia. These conditions worsen with gestational age and pose risks of eclampsia, DIC, and hepatic rupture. The only definitive resolution is placental removal, which halts the pathological cascade. Delivery is indicated regardless of gestational age once maternal or fetal compromise occurs.
Rationale for correct answer
3. Delivery of the placenta is the definitive treatment because it removes the source of antiangiogenic factors and inflammatory mediators. This halts endothelial damage and reverses systemic effects. In HELLP syndrome, delivery is urgent if gestational age is ≥34 weeks or maternal status deteriorates. It is the only intervention that stops disease progression.
Rationale for incorrect answers
1. Administration of corticosteroids may improve fetal lung maturity and transiently stabilize maternal labs, but it is not curative. Steroids do not reverse endothelial injury or halt placental factor release. They are adjunctive, not definitive treatment.
2. Strict bed rest does not alter the underlying pathophysiology. It may reduce blood pressure transiently but does not prevent progression to eclampsia, DIC, or hepatic rupture. Bed rest is outdated and not evidence-based as a primary intervention.
4. Long-term antihypertensive therapy manages blood pressure but does not treat the root cause—placental dysfunction. It may reduce stroke risk but cannot prevent worsening hemolysis, liver injury, or thrombocytopenia. Antihypertensives are supportive, not curative.
Take home points
- Delivery of the placenta is the only definitive treatment for preeclampsia and HELLP syndrome.
- Corticosteroids are supportive for fetal lung maturity, not curative.
- HELLP syndrome requires urgent delivery if maternal or fetal compromise occurs.
- Antihypertensives and bed rest do not reverse the disease process.
Which of the following are appropriate nursing interventions for a patient with HELLP syndrome? Select all that apply
Explanation
HELLP syndrome is a severe pregnancy complication involving hemolysis, elevated liver enzymes, and low platelet count. It results from endothelial dysfunction, microvascular thrombosis, and hepatic inflammation. Platelet consumption leads to bleeding risk, while liver injury causes right upper quadrant pain and elevated AST/ALT. Fluid shifts and capillary leak may cause pulmonary edema, necessitating fluid restriction. Monitoring for bleeding, liver function, and renal status is essential. Delivery is the definitive treatment, and supportive care includes transfusions, corticosteroids, and seizure prophylaxis.
Rationale for correct answers
1. Monitoring for signs of bleeding is critical due to thrombocytopenia and risk of DIC. Platelet counts <100,000/mm³ increase hemorrhage risk. Nurses must assess for petechiae, bruising, hematuria, and vaginal bleeding.
4. Restricting fluid intake helps prevent pulmonary edema, a complication of HELLP due to capillary leak and reduced oncotic pressure. Fluid overload worsens respiratory distress and increases maternal morbidity.
5. Monitoring liver function tests is essential because hepatic injury is a core feature. AST and ALT levels >70 IU/L indicate hepatocellular damage. Rising values may signal impending hepatic rupture or worsening inflammation.
Rationale for incorrect answers
2. Administering insulin is not indicated unless the patient has gestational diabetes. HELLP syndrome does not involve glucose dysregulation. Insulin has no role in managing hemolysis, liver injury, or thrombocytopenia.
3. Encouraging ambulation is contraindicated due to bleeding risk and potential for vascular instability. Patients may have low platelets and be at risk for falls, hemorrhage, or worsening symptoms. Bed rest with close monitoring is preferred.
Take home points
- HELLP syndrome requires close monitoring for bleeding and liver function deterioration.
- Fluid restriction prevents pulmonary edema due to capillary leak.
- Insulin is not part of HELLP management unless diabetes coexists.
- Ambulation is avoided due to bleeding and hemodynamic risks.
Practice Exercise 6
A nurse is caring for a patient with eclampsia. What is the priority action during a seizure?
Explanation
Eclampsia is a severe complication of preeclampsia characterized by new-onset tonic-clonic seizures in a pregnant patient with hypertension and proteinuria. It results from cerebral vasospasm, endothelial dysfunction, and cerebral edema. Common signs include headache, visual disturbances, and hyperreflexia. Seizures may lead to hypoxia, aspiration, and maternal-fetal injury. Blood pressure is typically ≥160/110 mmHg, and proteinuria exceeds 300 mg/24 hr. Magnesium sulfate is the first-line anticonvulsant. Immediate seizure management focuses on airway protection and injury prevention.
Rationale for correct answer
2. During an eclamptic seizure, the most critical intervention is to maintain airway patency and prevent trauma. Seizures compromise ventilation and increase aspiration risk. The nurse must position the patient in left lateral decubitus, clear the airway, and pad bedrails. This action directly prevents hypoxic injury and maternal-fetal compromise.
Rationale for incorrect answers
1. Antihypertensive medication is important in eclampsia, but not the priority during an active seizure. Blood pressure control is secondary to airway protection and seizure cessation. Administering labetalol or hydralazine is appropriate after stabilization. Giving medication during convulsions risks aspiration and is not immediately life-saving.
3. A non-stress test evaluates fetal well-being but is irrelevant during a seizure. The fetus may experience transient bradycardia due to maternal hypoxia. However, maternal stabilization takes precedence. Fetal monitoring is deferred until the mother is stable and seizure activity has ceased.
4. Corticosteroids are used to accelerate fetal lung maturity in preterm gestation, especially before 34 weeks. However, they do not address seizure control or maternal safety. Administering steroids during a seizure is not feasible and does not mitigate immediate risks like hypoxia or aspiration.
Take home points
- Eclampsia is defined by seizures in a hypertensive pregnant patient with preeclampsia.
- Airway protection and safety are the first priorities during convulsions.
- Magnesium sulfate is the drug of choice for seizure control in eclampsia.
- Antihypertensives and corticosteroids are secondary interventions after stabilization.
What is the primary cause of seizures in eclampsia?
Explanation
Eclampsia is a life-threatening obstetric emergency defined by new-onset generalized seizures in a patient with preeclampsia. The pathophysiology involves cerebral vasospasm, endothelial dysfunction, and blood-brain barrier disruption, leading to cerebral edema and ischemia. These changes result in increased intracranial pressure and neuronal excitability. Common symptoms include headache, visual disturbances, and hyperreflexia. MRI may show posterior reversible encephalopathy syndrome (PRES). Magnesium sulfate is the first-line anticonvulsant. Blood pressure is often ≥160/110 mmHg.
Rationale for correct answer
2. Seizures in eclampsia are primarily caused by cerebral vasospasm and edema, which impair cerebral perfusion and increase neuronal excitability. Vasospasm leads to ischemia, while edema disrupts the blood-brain barrier. These changes provoke cortical irritation and seizure activity. PRES is a common radiologic finding confirming this mechanism.
Rationale for incorrect answers
1. Hypoglycemia can cause seizures, but it is not the mechanism in eclampsia. Blood glucose levels in eclampsia are typically normal. The seizures are due to vascular and neurological changes, not metabolic derangements. Hypoglycemia-induced seizures present differently and are not associated with hypertension or proteinuria.
3. Electrolyte imbalance, such as hyponatremia or hypernatremia, may cause seizures, but this is not the primary cause in eclampsia. Serum electrolytes are usually within normal limits unless complicated by renal dysfunction. The dominant pathology is cerebral edema, not ionic shifts.
4. Hypocalcemia can cause tetany and seizures, but it is not implicated in eclampsia. Calcium levels are not typically low in preeclampsia or eclampsia. The seizures stem from vascular instability and brain edema, not calcium deficiency.
Take home points
- Eclampsia seizures result from cerebral vasospasm and edema, not metabolic causes.
- PRES is a common radiologic finding in eclampsia.
- Magnesium sulfate is the treatment of choice for seizure control.
- Electrolyte and glucose levels are usually normal in eclampsia.
Which of the following interventions are appropriate for a patient with eclampsia during a seizure? Select all that apply
Explanation
Eclampsia is a hypertensive disorder of pregnancy marked by new-onset tonic-clonic seizures in a patient with preeclampsia. It results from cerebral vasospasm, endothelial dysfunction, and blood-brain barrier disruption, leading to cerebral edema and neuronal hyperexcitability. The condition is associated with severe hypertension (≥160/110 mmHg), proteinuria (>300 mg/24 hr), and symptoms such as headache, visual disturbances, and hyperreflexia. The cornerstone of management includes airway protection, seizure control with magnesium sulfate, and blood pressure stabilization. MRI may reveal posterior reversible encephalopathy syndrome (PRES).
Rationale for correct answers
1. Turning the patient to the side reduces the risk of aspiration by allowing secretions to drain from the mouth and improves venous return by relieving pressure on the inferior vena cava. This position also helps maintain airway patency during convulsions.
3. Administering magnesium sulfate is the first-line treatment for seizure control in eclampsia. It acts as a central nervous system depressant, reducing neuronal excitability and preventing recurrent seizures. The loading dose is typically 4 g IV over 20 minutes, followed by a maintenance infusion.
4. Protecting the airway is critical during a seizure to prevent hypoxia and aspiration. This includes positioning, suctioning secretions, and providing supplemental oxygen. Airway protection takes precedence over all other interventions during active convulsions.
Rationale for incorrect answers
2. Restraining the patient’s limbs is contraindicated during a seizure. It increases the risk of musculoskeletal injury and does not prevent seizure activity. Instead, the nurse should ensure a safe environment by padding bedrails and removing nearby objects without physically restraining the patient.
5. Inserting an oral airway during an active seizure is dangerous and should be avoided. Attempting to place anything in the mouth during convulsions can cause oral trauma or airway obstruction. Airway adjuncts should only be inserted after the seizure has ended and the jaw is relaxed.
Take home points
- Eclampsia seizures require immediate airway protection and lateral positioning.
- Magnesium sulfate is the drug of choice for seizure control.
- Physical restraints and oral airways are contraindicated during active convulsions.
- PRES is a common radiologic finding in eclampsia-related seizures.
Magnesium sulfate is commonly administered to pregnant women with preeclampsia for which primary purpose?
Explanation
Magnesium sulfate is a centrally acting anticonvulsant used in obstetrics primarily for seizure prophylaxis in preeclampsia and treatment of eclampsia. It reduces neuromuscular excitability by blocking NMDA receptors and decreasing acetylcholine release at the neuromuscular junction. It does not lower blood pressure or induce labor. The loading dose is typically 4 g IV over 20 minutes followed by a 1 g/hr maintenance infusion. Toxicity signs include loss of deep tendon reflexes, respiratory depression, and cardiac arrest. Therapeutic serum magnesium level is 4.8–8.4 mg/dL.
Rationale for correct answer
3. Magnesium sulfate is administered to prevent seizures in patients with preeclampsia and to treat seizures in eclampsia. It stabilizes neuronal membranes and reduces cortical irritability. It is not an antihypertensive or uterotonic agent. Its primary role is neuroprotection.
Rationale for incorrect answers
1. Magnesium sulfate does not lower blood pressure. It has mild vasodilatory effects but is not used for hypertension control. Labetalol, hydralazine, or nifedipine are preferred antihypertensives in preeclampsia. Magnesium’s role is anticonvulsant, not antihypertensive.
2. Magnesium sulfate does not induce labor. In fact, it may cause uterine relaxation and delay contractions. Oxytocin or prostaglandins are used for labor induction. Magnesium is used to prevent seizures, not to initiate delivery.
4. Magnesium sulfate does not enhance fetal lung maturity. Corticosteroids such as betamethasone are used for that purpose before 34 weeks gestation. Magnesium may offer neuroprotection to the fetus but does not accelerate lung development.
Take home points
- Magnesium sulfate prevents seizures in preeclampsia and treats seizures in eclampsia.
- It does not lower blood pressure or induce labor.
- Toxicity signs include respiratory depression and absent reflexes.
- Corticosteroids—not magnesium—enhance fetal lung maturity.
When providing care for a client receiving magnesium sulfate for preeclampsia, which of the following assessments are essential to monitor for potential toxicity? Select all that apply
Explanation
Magnesium sulfate toxicity is a critical concern when administering this anticonvulsant for seizure prophylaxis in preeclampsia. Magnesium acts as a neuromuscular depressant, and excessive levels can suppress deep tendon reflexes, impair respiratory drive, and cause renal retention. Toxicity typically occurs when serum magnesium exceeds 9 mg/dL. Early signs include loss of patellar reflexes, followed by respiratory depression and cardiac arrest. Magnesium is renally excreted, so urine output must be monitored closely. Calcium gluconate is the antidote for toxicity.
Rationale for correct answers
1. Monitoring deep tendon reflexes is essential because their absence is an early sign of magnesium toxicity. The patellar reflex is typically assessed every hour. Reflex suppression indicates excessive neuromuscular blockade and precedes respiratory compromise.
2. Urine output is critical because magnesium is excreted renally. Output <30 mL/hr increases the risk of accumulation and toxicity. Renal impairment or oliguria necessitates dose adjustment or discontinuation.
4. Respiratory rate must be monitored because magnesium depresses the respiratory center. A rate <12 breaths/min suggests impending respiratory failure. Continuous pulse oximetry and respiratory assessment are mandatory during infusion.
Rationale for incorrect answers
3. Blood glucose levels are not affected by magnesium sulfate. Hypoglycemia is not a side effect of magnesium therapy. Glucose monitoring is relevant in gestational diabetes or insulin therapy, not in seizure prophylaxis with magnesium.
5. Fetal heart rate baseline variability is not a direct indicator of maternal magnesium toxicity. While magnesium may cause mild fetal sedation, it does not reliably reflect maternal serum levels or toxicity. Fetal monitoring is important but not a toxicity marker.
Take home points
- Magnesium sulfate toxicity presents with absent reflexes, respiratory depression, and reduced urine output.
- Monitor reflexes, respiratory rate, and urine output hourly during infusion.
- Calcium gluconate is the antidote for magnesium toxicity.
- Fetal heart rate variability is not a reliable marker of maternal toxicity.
Practice Exercise 7
Which of the following are maternal complications of hypertensive disorders in pregnancy? Select all that apply
Explanation
Hypertensive disorders in pregnancy are a spectrum of conditions including gestational hypertension, preeclampsia, eclampsia, and chronic hypertension. These disorders result from vasospasm, endothelial dysfunction, placental ischemia, and systemic inflammation. They lead to multi-organ damage affecting the kidneys, lungs, brain, and liver. Severe preeclampsia may present with systolic blood pressure ≥160 mmHg or diastolic ≥110 mmHg, proteinuria >300 mg/24h, elevated liver enzymes, thrombocytopenia <100,000/μL, and signs of end-organ damage. Eclampsia involves seizures. HELLP syndrome includes hemolysis, elevated liver enzymes, and low platelets. These conditions increase maternal morbidity and mortality.
Rationale for correct answers
1. Pulmonary edema is a recognized complication of severe preeclampsia due to capillary leak and fluid overload. Endothelial dysfunction increases vascular permeability, and aggressive fluid resuscitation or cardiac dysfunction can precipitate pulmonary edema. It presents with dyspnea, hypoxia, and crackles on auscultation.
2. Acute renal failure occurs due to renal vasoconstriction and glomerular endotheliosis. Preeclampsia reduces renal perfusion and glomerular filtration rate. Serum creatinine rises above 1.1 mg/dL or doubles from baseline. Oliguria <500 mL/day may be seen. Renal biopsy shows swollen glomeruli and fibrin deposition.
4. Cerebral hemorrhage results from severe hypertension and vascular rupture. Systolic pressures >160 mmHg increase risk of intracranial bleeding. Neurological signs include headache, visual disturbances, seizures, and altered consciousness. CT scan confirms hemorrhage. It is a leading cause of maternal death in eclampsia.
Rationale for incorrect answers
3. Gestational diabetes is not a complication of hypertensive disorders but a separate condition caused by placental hormones inducing insulin resistance. It typically presents in the second trimester and is diagnosed via oral glucose tolerance test. Although both conditions may coexist, they have distinct pathophysiology.
5. Hyperemesis gravidarum is unrelated to hypertension. It is a disorder of early pregnancy characterized by severe nausea and vomiting, dehydration, and electrolyte imbalance. It is linked to elevated human chorionic gonadotropin levels and often resolves by week 20. It does not involve vascular pathology or organ damage.
Take home points
- Hypertensive disorders in pregnancy can cause multi-organ complications including pulmonary, renal, and cerebral damage.
- Preeclampsia and eclampsia involve endothelial dysfunction and vasospasm leading to systemic effects.
- Gestational diabetes and hyperemesis gravidarum are not vascular complications and have distinct mechanisms.
- Cerebral hemorrhage is a life-threatening emergency requiring immediate blood pressure control and neuroimaging.
Which of the following are symptoms of severe preeclampsia? Select all that apply
Explanation
Severe preeclampsia is a hypertensive disorder of pregnancy characterized by endothelial dysfunction, vasospasm, placental ischemia, and multi-organ involvement. It typically presents after 20 weeks gestation with systolic blood pressure ≥160 mmHg or diastolic ≥110 mmHg, proteinuria >5 g/day, or signs of end-organ damage. These include elevated liver enzymes, thrombocytopenia <100,000/μL, serum creatinine >1.1 mg/dL, pulmonary edema, and neurological symptoms. The pathophysiology involves abnormal placentation, leading to systemic inflammation and vascular injury. It increases risk for maternal stroke, renal failure, and fetal growth restriction.
Rationale for correct answers
1. Visual disturbances such as scotomata or blurred vision indicate cerebral involvement due to vasospasm or cerebral edema. These are warning signs of impending eclampsia and require urgent evaluation. They reflect central nervous system irritation and are part of diagnostic criteria for severe preeclampsia.
2. Epigastric pain is caused by hepatic capsule distension due to periportal hemorrhage or subcapsular hematoma. It reflects liver involvement and is often associated with elevated transaminases. This symptom may precede HELLP syndrome and is a red flag for disease progression.
4. Oliguria, defined as urine output <500 mL/day, results from renal vasoconstriction and glomerular endotheliosis. It indicates reduced renal perfusion and is a marker of severe disease. Serum creatinine may rise, and fluid balance must be closely monitored.
Rationale for incorrect answers
3. Mild hypertension, defined as systolic 140–159 mmHg or diastolic 90–109 mmHg, is not a feature of severe preeclampsia. Severe disease requires systolic ≥160 mmHg or diastolic ≥110 mmHg. Mild hypertension may be seen in gestational hypertension or mild preeclampsia without end-organ damage.
5. Increased fetal movement is not a symptom of preeclampsia. In fact, decreased fetal movement is more concerning and may indicate fetal hypoxia or growth restriction due to uteroplacental insufficiency. Fetal hyperactivity is not a diagnostic criterion for hypertensive disorders in pregnancy.
Take home points
- Severe preeclampsia presents with end-organ dysfunction including CNS, renal, and hepatic involvement.
- Visual changes and epigastric pain are red flags for progression to eclampsia or HELLP syndrome.
- Oliguria reflects renal compromise and requires close fluid and renal monitoring.
- Mild hypertension and increased fetal movement are not diagnostic of severe preeclampsia.
Which of the following are potential fetal complications of hypertensive disorders? Select all that apply
Explanation
Fetal complications of hypertensive disorders arise due to placental insufficiency, vasospasm, reduced uteroplacental perfusion, and chronic hypoxia. These pathophysiological changes impair nutrient and oxygen delivery to the fetus, leading to growth restriction, prematurity, and hypoxic injury. The placenta may show infarcts, fibrin deposition, and abnormal villous development. Doppler studies reveal elevated umbilical artery resistance. Fetal surveillance includes biophysical profile, non-stress test, and umbilical artery Doppler. Severe maternal hypertension increases risk of fetal demise, low birth weight <2500 g, and Apgar scores <7 at 5 minutes.
Rationale for correct answers
1. Intrauterine growth restriction (IUGR) results from chronic placental hypoperfusion and nutrient deprivation. Hypertensive disorders impair trophoblastic invasion and spiral artery remodeling, reducing placental blood flow. Fetal abdominal circumference and estimated weight fall below the 10th percentile.
2. Preterm birth is common due to iatrogenic delivery for maternal or fetal indications. Severe preeclampsia or eclampsia may necessitate early termination to prevent maternal complications. Spontaneous preterm labor may also occur due to placental inflammation or abruption.
4. Fetal hypoxia occurs due to reduced oxygen delivery from impaired uteroplacental circulation. Chronic hypoxia leads to abnormal fetal heart rate patterns, meconium-stained amniotic fluid, and low Apgar scores. Doppler velocimetry may show absent or reversed end-diastolic flow.
Rationale for incorrect answers
3. Macrosomia is not associated with hypertensive disorders. It is typically seen in gestational diabetes due to fetal hyperinsulinemia and excessive glucose transfer. Hypertensive pregnancies more commonly result in growth restriction, not excessive growth.
5. Congenital anomalies are not directly caused by hypertensive disorders. Structural malformations arise from genetic defects or teratogenic exposures during organogenesis. Hypertension affects placental function but does not interfere with embryonic development in early gestation.
Take home points
- Hypertensive disorders impair placental perfusion, leading to fetal growth restriction and hypoxia.
- Preterm birth often results from early delivery due to maternal or fetal compromise.
- Macrosomia is linked to gestational diabetes, not hypertension.
Comprehensive Questions
What is the definitive treatment for preeclampsia?
Explanation
Definitive treatment of preeclampsia involves terminating the pregnancy to eliminate the source of the disease process. Preeclampsia is a placenta-driven disorder characterized by hypertension, proteinuria, and end-organ dysfunction after 20 weeks gestation. The pathophysiology involves abnormal placentation, leading to systemic endothelial dysfunction and vasospasm. The only cure is delivery, which removes the placenta and halts disease progression. Management depends on gestational age and severity. Severe features include BP ≥160/110 mmHg, thrombocytopenia <100,000/μL, elevated liver enzymes, and pulmonary edema. Magnesium sulfate is used for seizure prophylaxis, and antihypertensives control BP temporarily, but neither cures the condition.
Rationale for correct answer
3. Delivery of the fetus is the definitive treatment because it removes the placental source of the disease. Preeclampsia is a pregnancy-specific condition that resolves only after placental separation. The question asks for the definitive treatment, not supportive management, making delivery the only correct answer.
Rationale for incorrect answers
1. Antihypertensive therapy is used to reduce blood pressure and prevent complications like stroke, but it does not treat the underlying placental pathology. Drugs like labetalol or hydralazine are supportive, not curative. BP control alone does not halt disease progression.
2. Magnesium sulfate prevents seizures but does not reverse the disease process. It is used for neuroprotection and eclampsia prevention, not as a curative measure. The underlying endothelial dysfunction persists unless the placenta is removed.
4. Corticosteroids are used to accelerate fetal lung maturity in preterm gestations but have no effect on the maternal disease process. They are adjunctive therapy to improve neonatal outcomes, not definitive treatment for preeclampsia.
Take home points
- Delivery is the only cure for preeclampsia, especially with severe features.
- Magnesium sulfate prevents seizures but does not treat the disease.
- Antihypertensives reduce BP but do not reverse placental pathology.
- Corticosteroids improve fetal lung maturity but do not affect maternal disease.
Which blood pressure reading indicates severe preeclampsia?
Explanation
Severe preeclampsia is defined by specific clinical and laboratory criteria that indicate significant maternal risk. It involves sustained hypertension, end-organ damage, placental dysfunction, and neurologic symptoms. The hallmark is blood pressure ≥160/110 mmHg measured on two occasions at least 4 hours apart. Additional features include thrombocytopenia <100,000/μL, serum creatinine >1.1 mg/dL or doubling of baseline, elevated liver transaminases (AST or ALT >2× normal), pulmonary edema, and cerebral or visual disturbances. Vasospasm, endothelial injury, ischemia, and coagulopathy underlie the pathophysiology. Management includes seizure prophylaxis, BP control, and expedited delivery depending on gestational age and maternal-fetal status.
Rationale for correct answer
3. A reading of 160/110 mmHg meets the threshold for severe hypertension, one of the diagnostic criteria for severe preeclampsia. This level of pressure increases the risk of stroke, placental abruption, and eclampsia. The question specifically asks for the BP value that defines severity, making this the correct choice.
Rationale for incorrect answers
1. A BP of 140/90 mmHg meets the minimum threshold for gestational hypertension or mild preeclampsia, but not severe disease. It does not fulfill the criteria for severe features and is managed conservatively unless other symptoms develop.
2. A BP of 150/100 mmHg is elevated but does not meet the severe range. It may indicate worsening disease but is not diagnostic of severe preeclampsia. Close monitoring is warranted, but it does not trigger immediate intervention.
4. A BP of 130/80 mmHg is within normal limits for pregnancy. It does not meet any diagnostic criteria for hypertensive disorders in pregnancy. No clinical concern for preeclampsia arises from this value alone.
Take home points
- Severe preeclampsia is defined by BP ≥160/110 mmHg and signs of end-organ damage.
- Mild preeclampsia starts at BP ≥140/90 mmHg with proteinuria or other features.
- Normal pregnancy BP is typically <140/90 mmHg.
A client with preeclampsia reports new onset of severe, persistent headache unrelieved by acetaminophen. The nurse recognizes this symptom as a potential sign of:
Explanation
Worsening preeclampsia is a progression of preeclampsia marked by new or intensifying symptoms indicating end-organ dysfunction. It results from systemic vasospasm, endothelial injury, cerebral edema, and impaired perfusion. Severe features include persistent headache, visual disturbances, right upper quadrant pain, pulmonary edema, and thrombocytopenia. The headache is due to cerebral vasoconstriction and increased intracranial pressure. These symptoms are red flags for imminent eclampsia or stroke. Blood pressure is typically ≥160/110 mmHg. Immediate evaluation and stabilization with magnesium sulfate and antihypertensives are required, followed by delivery if maternal or fetal compromise is present.
Rationale for correct answer
3. A new, severe, persistent headache unrelieved by acetaminophen is a hallmark of cerebral involvement in worsening preeclampsia. It reflects neurologic irritability and increased risk of seizures. This symptom requires urgent evaluation and treatment to prevent progression to eclampsia or stroke.
Rationale for incorrect answers
1. While mild headaches can occur in normal pregnancy, a severe, persistent headache that does not respond to analgesics is not typical. Normal pregnancy discomforts are usually transient and mild. This presentation exceeds expected physiologic changes.
2. Dehydration may cause headache, but it is usually associated with other signs like dry mucous membranes, tachycardia, and decreased urine output. It typically responds to hydration and rest. The persistence and severity here suggest a more serious etiology.
4. Hypoglycemia can cause headache, but it is usually accompanied by symptoms like diaphoresis, tremors, hunger, and confusion. It resolves quickly with glucose intake. The absence of these features and lack of response to acetaminophen makes this unlikely.
Take home points
- Severe headache in preeclampsia signals cerebral involvement and risk of eclampsia.
- Normal pregnancy headaches are usually mild and transient.
- Dehydration and hypoglycemia have distinct symptom clusters.
- Worsening preeclampsia requires immediate intervention to prevent maternal complications.
Which fetal complication is associated with hypertensive disorders in pregnancy?
Explanation
Hypertensive disorders in pregnancy are a group of conditions characterized by elevated blood pressure after 20 weeks gestation. Vasospasm, endothelial dysfunction, placental hypoperfusion, and uteroplacental insufficiency are central mechanisms. These lead to reduced oxygen and nutrient delivery to the fetus, causing complications. Systolic ≥140 mmHg or diastolic ≥90 mmHg defines hypertension. Severe features include proteinuria ≥300 mg/24 hr, elevated liver enzymes, and thrombocytopenia. Chronic hypertension, gestational hypertension, preeclampsia, and eclampsia are subtypes. Fetal effects depend on severity and duration of maternal hypertension.
Rationale for correct answer
2. Intrauterine growth restriction results from placental insufficiency due to vasospasm and impaired trophoblastic invasion, which are hallmark features of hypertensive disorders. The fetus receives inadequate oxygen and nutrients, leading to reduced growth velocity. Doppler studies often show abnormal umbilical artery flow. This is the most consistent fetal complication associated with maternal hypertension.
Rationale for incorrect answers
1. Macrosomia is typically associated with maternal diabetes due to fetal hyperinsulinemia and increased glucose transfer. Hypertensive disorders do not cause fetal overgrowth; instead, they restrict growth. In fact, the opposite—low birth weight—is more common in hypertensive pregnancies.
3. Polyhydramnios is linked to fetal anomalies such as anencephaly or gastrointestinal obstructions, and maternal diabetes. Hypertensive disorders often result in oligohydramnios due to reduced placental perfusion and decreased fetal urine output, not excess amniotic fluid.
4. Congenital heart defects are primarily associated with teratogenic exposures or genetic syndromes. Hypertension in pregnancy does not directly cause structural cardiac malformations. There is no strong evidence linking maternal hypertension to increased incidence of congenital heart anomalies.
Take home points
- Hypertensive disorders impair placental perfusion, leading to fetal growth restriction.
- Macrosomia is a complication of maternal diabetes, not hypertension.
- Polyhydramnios is more common in fetal anomalies and diabetes, not hypertensive states.
- Congenital heart defects are not directly caused by maternal hypertension.
When administering magnesium sulfate, the nurse must closely monitor the client for signs of toxicity. Which of the following is an early sign of magnesium toxicity?
Explanation
Magnesium sulfate toxicity occurs when serum magnesium levels exceed the therapeutic range, typically above 8 mEq/L. Magnesium sulfate is used for seizure prophylaxis in preeclampsia and eclampsia. Neuromuscular depression, respiratory compromise, hypotension, and cardiac conduction delay are hallmark features of toxicity. Early signs include diminished deep tendon reflexes, progressing to respiratory depression below 12 breaths/minute and cardiac arrest. Therapeutic serum magnesium levels range from 4 to 7 mEq/L. Calcium gluconate is the antidote.
Rationale for correct answer
3. Loss of deep tendon reflexes is the earliest reliable clinical indicator of magnesium toxicity. Magnesium acts as a calcium antagonist at neuromuscular junctions, leading to reflex suppression. The question stem emphasizes monitoring for early signs, and reflex loss precedes respiratory depression and cardiac effects. This is a direct sign of neuromuscular blockade.
Rationale for incorrect answers
1. Increased deep tendon reflexes are not associated with magnesium toxicity. In fact, hyperreflexia is seen in early preeclampsia before magnesium is administered. Magnesium causes reflex depression, not enhancement. This choice contradicts the pharmacologic action of magnesium on neuromuscular transmission.
2. Respiratory rate of 20 breaths/minute is within normal limits. Toxic levels of magnesium depress the respiratory center, leading to bradypnea below 12 breaths/minute. A rate of 20 indicates adequate ventilation and no current compromise. This is not an early sign of toxicity.
4. Hypertension resistant to medication is not a feature of magnesium toxicity. Magnesium sulfate causes vasodilation and lowers blood pressure. Persistent hypertension may indicate worsening preeclampsia, not magnesium overdose. This reflects disease progression, not drug toxicity.
Take home points
- Loss of deep tendon reflexes is the earliest sign of magnesium toxicity.
- Magnesium sulfate toxicity causes neuromuscular and respiratory depression.
- Therapeutic magnesium levels range from 4 to 7 mEq/L.
- Calcium gluconate is the antidote for magnesium toxicity.
A client with HELLP syndrome is found to have a platelet count of 15,000/mm3. The healthcare provider orders an immediate Cesarean section. What is a crucial consideration for the nursing team regarding anesthesia?
Explanation
Anesthesia in thrombocytopenia requires careful evaluation of bleeding risk, especially in obstetric emergencies like HELLP syndrome. Platelet count, coagulation status, risk of spinal hematoma, and urgency of delivery guide anesthetic choice. Neuraxial techniques (spinal or epidural) are contraindicated when platelets fall below 75,000/mm³ due to risk of epidural hematoma and neurologic injury. In severe thrombocytopenia (<20,000/mm³), general anesthesia is preferred to avoid central neuraxial bleeding. HELLP syndrome often necessitates rapid delivery, increasing reliance on general anesthesia.
Rationale for correct answer
2. General anesthesia may be safer due to the critically low platelet count of 15,000/mm³. Neuraxial techniques are contraindicated below 75,000/mm³ due to risk of epidural hematoma. General anesthesia avoids spinal canal instrumentation and minimizes risk of neurologic complications. The urgency of Cesarean delivery further supports this approach.
Rationale for incorrect answers
1. Spinal anesthesia is contraindicated at platelet counts below 75,000/mm³. At 15,000/mm³, the risk of spinal bleeding and compression is unacceptably high. This choice ignores standard safety thresholds and exposes the patient to irreversible neurologic injury.
3. Epidural anesthesia is not safe at platelet counts below 75,000/mm³. It carries similar risks to spinal anesthesia, including epidural hematoma and paralysis. The slower onset of epidural also makes it unsuitable for emergent delivery.
4. No anesthesia is never appropriate, even in emergencies. Cesarean section is a major abdominal surgery requiring pain control and muscle relaxation. Proceeding without anesthesia would cause severe distress and violate ethical standards of care.
Take home points
- General anesthesia is preferred in HELLP syndrome with platelets <75,000/mm³.
- Neuraxial anesthesia is contraindicated due to risk of epidural hematoma.
- HELLP syndrome may require urgent delivery, influencing anesthesia choice.
- Platelet count guides safe anesthesia modality in obstetric emergencies.
Which anti-angiogenic factor is implicated in the pathophysiology of preeclampsia?
Explanation
Preeclampsia is a hypertensive disorder of pregnancy characterized by endothelial dysfunction, placental ischemia, anti-angiogenic imbalance, and systemic inflammation. The placenta releases excess soluble fms-like tyrosine kinase-1 (sFlt-1), which binds and neutralizes vascular endothelial growth factor (VEGF) and placental growth factor (PlGF). This leads to impaired angiogenesis, vasoconstriction, and increased vascular permeability. Clinical features include hypertension after 20 weeks, proteinuria >300 mg/day, and organ dysfunction. Severe cases may progress to HELLP syndrome or eclampsia.
Rationale for correct answer
2. sFlt-1 is a soluble receptor that antagonizes VEGF and PlGF, disrupting angiogenesis. Elevated levels of sFlt-1 in maternal circulation are a hallmark of preeclampsia. It causes endothelial injury and vasoconstriction, leading to hypertension and proteinuria. Its role in neutralizing pro-angiogenic factors makes it central to the disease mechanism.
Rationale for incorrect answers
1. Vascular endothelial growth factor is a pro-angiogenic molecule that promotes vascular development and endothelial integrity. In preeclampsia, VEGF is suppressed by sFlt-1, not elevated. Its deficiency contributes to vascular dysfunction, not its presence. This factor is not the pathogenic agent but a target of inhibition.
3. Placental growth factor is another pro-angiogenic molecule essential for placental vascularization. Like VEGF, it is neutralized by sFlt-1 in preeclampsia. Low levels of PlGF are associated with disease severity, but it is not the causative factor. It is a victim of antagonism, not the driver.
4. Insulin-like growth factor is involved in fetal growth and placental development but is not directly implicated in the angiogenic imbalance of preeclampsia. It does not interact with VEGF or PlGF pathways. Its role is metabolic, not vascular, making it irrelevant to the anti-angiogenic mechanism.
Take home points
- sFlt-1 is the key anti-angiogenic factor in preeclampsia.
- VEGF and PlGF are suppressed in preeclampsia due to sFlt-1 elevation.
- Endothelial dysfunction drives hypertension and proteinuria.
- Preeclampsia arises from placental ischemia and angiogenic imbalance.
Why is accurate blood pressure measurement particularly important in the diagnosis and management of hypertensive disorders of pregnancy?
Explanation
Hypertensive disorders in pregnancy include gestational hypertension, preeclampsia, eclampsia, and chronic hypertension. Accurate blood pressure monitoring, severity classification, timing of delivery, and treatment decisions are central to management. Diagnostic thresholds are ≥140/90 mmHg for mild and ≥160/110 mmHg for severe hypertension. End-organ damage, proteinuria, neurologic symptoms, and fetal compromise guide escalation. Antihypertensives are initiated when systolic ≥160 mmHg or diastolic ≥110 mmHg. Magnesium sulfate is used for seizure prophylaxis in preeclampsia/eclampsia.
Rationale for correct answer
4. Accurate blood pressure measurement is essential to classify the severity of hypertensive disorders and guide treatment. Thresholds determine whether the condition is mild or severe, influencing decisions on antihypertensive therapy and timing of delivery. Misclassification can lead to delayed intervention or unnecessary escalation, affecting maternal and fetal outcomes.
Rationale for incorrect answers
1. Blood pressure does not directly determine fetal well-being. Fetal status is assessed via ultrasound, biophysical profile, and non-stress testing. While maternal hypertension may affect placental perfusion, BP alone is not a reliable indicator of fetal compromise.
2. Pain level is not assessed through blood pressure. Pain may transiently elevate BP, but accurate pain evaluation requires subjective reporting and behavioral cues. BP monitoring is not a validated tool for pain assessment in obstetrics.
3. Postpartum hemorrhage is influenced by uterine atony, retained placenta, and coagulopathy—not directly by blood pressure. While severe preeclampsia may predispose to bleeding, BP measurement is not a preventive tool for hemorrhage control.
Take home points
- Accurate BP guides classification and treatment of hypertensive disorders in pregnancy.
- Severe hypertension requires urgent antihypertensive therapy and possible delivery.
- BP does not directly assess fetal well-being or pain.
- Postpartum hemorrhage prevention involves uterine tone and coagulation, not BP.
Which of the following are signs of magnesium sulfate toxicity? Select all that apply
Explanation
Magnesium sulfate toxicity occurs when serum magnesium levels exceed the therapeutic range, typically >8 mEq/L. It is used in obstetrics for seizure prophylaxis in preeclampsia and eclampsia. Toxicity leads to neuromuscular depression, respiratory compromise, central nervous system suppression, and cardiac conduction abnormalities. Normal therapeutic serum magnesium is 4–7 mEq/L. Toxic effects begin above 8 mEq/L, with respiratory paralysis and cardiac arrest possible above 12 mEq/L. Early signs include loss of deep tendon reflexes, followed by respiratory depression and altered consciousness. Magnesium is renally excreted, so impaired renal function increases risk.
Rationale for correct answers
1. Loss of deep tendon reflexes is an early sign of magnesium toxicity due to neuromuscular blockade. Magnesium inhibits acetylcholine release at the neuromuscular junction, leading to diminished reflexes. This is often the first clinical indicator of rising magnesium levels.
2. Respiratory rate <12 breaths per minute indicates respiratory depression, a hallmark of magnesium toxicity. Magnesium suppresses the respiratory center in the medulla, leading to hypoventilation. This occurs as toxicity progresses beyond therapeutic levels.
4. Decreased level of consciousness reflects central nervous system depression caused by excess magnesium. High levels impair synaptic transmission and neuronal excitability, resulting in lethargy, confusion, and eventually coma.
Rationale for incorrect answers
3. Increased urine output is not a sign of magnesium toxicity. In fact, magnesium is excreted renally, and renal impairment increases toxicity risk. Polyuria is not associated with magnesium excess; oliguria or anuria may worsen toxicity due to reduced clearance.
5. Elevated blood pressure is not a feature of magnesium toxicity. Magnesium has vasodilatory effects, lowering systemic vascular resistance and blood pressure. In toxicity, hypotension is more likely due to smooth muscle relaxation and cardiovascular depression.
Take home points
- Magnesium sulfate toxicity presents with neuromuscular, respiratory, and CNS depression.
- Deep tendon reflexes are the earliest clinical sign of toxicity.
- Respiratory rate and consciousness level must be closely monitored during therapy.
- Renal function affects magnesium clearance and toxicity risk.
Immediate nursing interventions for a client experiencing an eclamptic seizure include which of the following? Select all that apply
Explanation
Eclampsia seizure management involves rapid stabilization of maternal physiology to prevent hypoxia, aspiration, and further neurologic injury. Eclampsia is defined as new-onset generalized tonic-clonic seizures in a woman with preeclampsia. The pathophysiology includes cerebral vasospasm, endothelial dysfunction, cerebral edema, and ischemia. Seizures may cause hypoxia, aspiration, and placental abruption. Magnesium sulfate is the drug of choice for seizure control. Airway protection and lateral positioning reduce aspiration risk. Blood pressure control and fetal monitoring follow stabilization.
Rationale for correct answers
2. Positioning the client on her side reduces the risk of aspiration and promotes venous return. Left lateral position is preferred to prevent aortocaval compression and facilitate airway patency during convulsions.
3. Ensuring the airway is patent is critical to prevent hypoxia and aspiration. Seizures compromise airway tone and protective reflexes, increasing risk of obstruction and pulmonary complications.
5. Administering magnesium sulfate as ordered is essential for seizure control and neuroprotection. Magnesium acts as a central nervous system depressant and stabilizes neuronal membranes, reducing seizure recurrence.
Rationale for incorrect answers
1. Administering a large bolus of IV fluids rapidly is contraindicated. Excessive fluid can worsen pulmonary edema due to endothelial leakage and reduced colloid osmotic pressure in preeclampsia. Fluid management must be cautious and guided by urine output and hemodynamics.
4. Restraining the client's movements to prevent injury is not recommended. Physical restraint increases risk of musculoskeletal trauma and does not prevent seizure-related injury. Instead, soft padding and side positioning are safer approaches.
Take home points
- Eclampsia requires immediate airway protection and seizure control.
- Magnesium sulfate is the first-line anticonvulsant in eclampsia.
- Lateral positioning reduces aspiration and improves maternal circulation.
- Aggressive fluid resuscitation and physical restraint are contraindicated.
Which of the following are components of HELLP syndrome? Select all that apply
Explanation
HELLP syndrome is a severe variant of preeclampsia characterized by Hemolysis, Elevated Liver enzymes, and Low Platelet count. It typically presents in the third trimester or postpartum period. The pathogenesis involves endothelial dysfunction, microangiopathic hemolytic anemia, hepatic injury, and platelet consumption. Hemolysis results from red blood cell fragmentation in narrowed vessels. Liver involvement leads to hepatocellular necrosis and elevated transaminases (AST >70 U/L, ALT elevated). Platelet count is typically <100,000/mm³. HELLP increases risk of disseminated intravascular coagulation, placental abruption, and maternal morbidity.
Rationale for correct answers
1. Hemolysis is a defining feature of HELLP syndrome. It results from microangiopathic hemolytic anemia, where red blood cells are damaged as they pass through narrowed, fibrin-laden vessels. Peripheral smear shows schistocytes.
2. Elevated liver enzymes reflect hepatocellular injury due to periportal necrosis and hepatic ischemia. AST and ALT levels are typically elevated, often >70 U/L, indicating liver involvement.
4. Low platelet count (<100,000/mm³) occurs due to platelet activation and consumption in the microvasculature. This thrombocytopenia increases bleeding risk and is a diagnostic criterion for HELLP.
Rationale for incorrect answers
3. Hypoglycemia is not a component of HELLP syndrome. While liver dysfunction may impair gluconeogenesis, clinically significant hypoglycemia is not characteristic. HELLP is associated with elevated liver enzymes, not glucose abnormalities.
5. Hypertension is common in preeclampsia but is not a diagnostic criterion for HELLP syndrome. While many patients with HELLP have elevated blood pressure, it is not required for diagnosis. Normotensive HELLP can occur.
Take home points
- HELLP stands for Hemolysis, Elevated Liver enzymes, and Low Platelets.
- It is a severe form of preeclampsia with high maternal-fetal risk.
- Hypertension may be present but is not diagnostic.
- Hypoglycemia is not a feature of HELLP syndrome.
Which of the following symptoms are indicative of severe preeclampsia? Select all that apply
Explanation
Severe preeclampsia is a hypertensive disorder of pregnancy marked by endothelial dysfunction, vasospasm, organ ischemia, and coagulopathy. It typically presents after 20 weeks gestation with systolic blood pressure ≥160 mmHg or diastolic ≥110 mmHg, and signs of end-organ damage. These include cerebral symptoms, hepatic involvement, renal impairment, and hematologic abnormalities. Proteinuria ≥300 mg/24h or a protein/creatinine ratio ≥0.3 may be present but is not required for diagnosis. Platelet count <100,000/mm³, elevated liver enzymes, and serum creatinine >1.1 mg/dL are diagnostic markers. Severe features necessitate urgent management to prevent eclampsia, stroke, or placental abruption.
Rationale for correct answers
1. Systolic blood pressure ≥160 mmHg meets the threshold for severe hypertension, a diagnostic criterion for severe preeclampsia. Sustained readings at or above this level increase risk of cerebral hemorrhage and require antihypertensive therapy.
2. Report of severe headache indicates cerebral involvement, a hallmark of severe preeclampsia. It reflects vasospasm and increased intracranial pressure, often preceding seizures or stroke.
5. Epigastric pain suggests hepatic capsular distension due to periportal necrosis or subcapsular hematoma. It is a sign of hepatic involvement, often accompanied by elevated liver enzymes and risk of hepatic rupture.
Rationale for incorrect answers
3. No proteinuria does not exclude severe preeclampsia. While proteinuria is a classic feature, diagnosis can be made based on end-organ damage alone. Absence of proteinuria does not rule out severity if other criteria are met.
4. Platelet count ≥100,000/mm³ is not indicative of severe disease. Severe preeclampsia includes thrombocytopenia with platelets <100,000/mm³ due to platelet consumption and endothelial activation. Normal platelet count suggests absence of hematologic severity.
Take home points
- Severe preeclampsia includes hypertension ≥160/110 mmHg and signs of end-organ damage.
- Proteinuria is not required for diagnosis if other severe features are present.
- Cerebral symptoms and epigastric pain indicate neurologic and hepatic involvement.
- Platelet count <100,000/mm³ is a marker of hematologic severity.
Which of the following are potential fetal complications of hypertensive disorders? Select all that apply
Explanation
Fetal complications of hypertensive disorders arise due to placental insufficiency, vasospasm, impaired uteroplacental perfusion, and endothelial dysfunction. These mechanisms reduce oxygen and nutrient delivery to the fetus, leading to intrauterine growth restriction, hypoxia, and preterm birth. Chronic hypertension, preeclampsia, and eclampsia compromise placental function, increasing risk of placental abruption, oligohydramnios, and fetal distress. Doppler studies may show absent or reversed end-diastolic flow in severe cases. Fetal surveillance includes biophysical profile, non-stress testing, and umbilical artery Doppler velocimetry.
Rationale for correct answers
1. Intrauterine growth restriction results from chronic placental hypoperfusion due to maternal vasospasm and endothelial dysfunction. Reduced nutrient and oxygen delivery impairs fetal growth, often seen with abnormal Doppler flow.
2. Preterm birth is common in hypertensive disorders due to iatrogenic delivery for maternal or fetal indications. Severe preeclampsia or eclampsia often necessitates early delivery to prevent maternal-fetal complications.
4. Fetal hypoxia occurs due to impaired oxygen exchange across the placenta. Vasoconstriction and placental infarction reduce oxygen availability, leading to acidosis, non-reassuring fetal heart patterns, and possible stillbirth.
Rationale for incorrect answers
3. Macrosomia is not associated with hypertensive disorders. It is linked to maternal diabetes, where excess glucose stimulates fetal insulin and growth. Hypertensive pregnancies typically show restricted growth, not overgrowth.
5. Congenital anomalies are not caused by hypertensive disorders. These conditions affect placental function, not embryogenesis. Structural anomalies are more associated with teratogenic exposures or genetic syndromes, not hypertension.
Take home points
- Hypertensive disorders impair placental perfusion, leading to fetal growth restriction and hypoxia.
- Preterm birth often results from early delivery due to maternal or fetal compromise.
- Macrosomia is linked to diabetes, not hypertension.
- Congenital anomalies are not caused by hypertensive disorders.
Exams on Hypertensive Disorders
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
- Objectives
- Introduction
- Classification Of Hypertensive Disorders Of Pregnancy
- Practice Exercise 1
- Pathophysiology Of Hypertensive Disorders
- Practice Exercise 2
- Clinical Manifestations And Diagnostic Findings
- Practice Exercise 3
- Management Of Hypertensive Disorders In Pregnancy
- Practice Exercise 4
- Hellp Syndrome (Hemolysis, Elevated Liver Enzymes, Low Platelet Count)
- Practice Exercise 5
- Eclampsia: Seizure Management And Nursing Care
- Practice Exercise 6
- Maternal And Fetal Complications Of Hypertensive Disorders
- Practice Exercise 7
- Summary
- Comprehensive Questions
Notes Highlighting is available once you sign in. Login Here.
Objectives
- Define and classify hypertensive disorders of pregnancy based on current obstetric standards, differentiating between gestational hypertension, preeclampsia (with and without severe features), eclampsia, chronic hypertension, and superimposed preeclampsia.
- Explain the pathophysiologic mechanisms underlying hypertensive disorders of pregnancy, including abnormal trophoblastic invasion, endothelial dysfunction, generalized vasospasm, coagulation activation, and the resultant multi-organ microangiopathy.
- Identify and interpret diagnostic criteria for hypertensive disorders in pregnancy, including blood pressure thresholds, proteinuria quantification, and laboratory parameters of organ involvement.
- Correlate fetal assessment findings such as growth restriction, oligohydramnios, and abnormal Doppler velocimetry with maternal disease severity and uteroplacental perfusion compromise.
- Describe the clinical manifestations of each hypertensive disorder, differentiating between mild and severe disease presentations and recognizing early warning signs of deterioration.
- Discuss pharmacologic management strategies, including the mechanisms of action, dosages, and nursing considerations for antihypertensive drugs (labetalol, hydralazine, nifedipine), anticonvulsant therapy (magnesium sulfate), and corticosteroids for fetal lung maturity.
- Explain the principles of magnesium sulfate therapy, including its pharmacodynamics, toxicity monitoring, therapeutic serum levels, and nursing interventions for prevention of magnesium toxicity.
- Discuss the obstetric management regarding timing and mode of delivery in patients with hypertensive disorders, considering maternal stability, gestational age, and fetal well-being.
- Explain the pathophysiology, diagnostic criteria, and management of HELLP Syndrome, emphasizing early recognition and the nursing priorities to prevent maternal morbidity and mortality.
- Describe the seizure mechanism in eclampsia, identify precipitating factors, and demonstrate step-by-step nursing care during and after a seizure to ensure maternal and fetal safety.
- Identify maternal and fetal complications arising from hypertensive disorders, including disseminated intravascular coagulation (DIC), placental abruption, intrauterine growth restriction (IUGR), preterm birth, and stillbirth.
- Provide patient-centered health education focusing on lifestyle modification, warning signs, adherence to medication, and follow-up care during the postpartum period.
Introduction
Hypertensive disorders of pregnancy (HDP) represent one of the most critical complications encountered in obstetrics, contributing substantially to maternal and perinatal morbidity and mortality worldwide. These disorders encompass a spectrum of clinical conditions characterized by elevated blood pressure during pregnancy, often accompanied by systemic involvement that affects multiple organ systems including the renal, hepatic, cerebral, and hematologic systems.
Definition
Hypertensive disorders of pregnancy are defined as conditions in which a pregnant woman develops systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg on at least two occasions, measured at least 4 hours apart after 20 weeks of gestation. These disorders are classified based on timing, severity, and the presence of proteinuria or end-organ dysfunction.
Epidemiology and Global Significance
HDPs occur in approximately 5%–10% of all pregnancies and are among the leading causes of maternal deaths globally, second only to hemorrhage. Preeclampsia and eclampsia together account for more than 70,000 maternal deaths and 500,000 perinatal deaths annually. The burden is particularly high in low-resource settings, where early detection and appropriate management are often limited.
Beyond immediate pregnancy outcomes, women who experience hypertensive disorders face an increased lifetime risk of chronic hypertension, cardiovascular disease, and renal impairment.
Etiologic Overview
The exact cause of hypertensive disorders of pregnancy remains multifactorial and incompletely understood. The most widely accepted theory implicates abnormal placentation during early gestation, leading to incomplete remodeling of the spiral arteries. This results in placental ischemia, endothelial dysfunction, and systemic vasospasm, which manifest clinically as hypertension and end-organ injury. Genetic, immunologic, and environmental factors also contribute to the pathogenesis.
Classification Of Hypertensive Disorders Of Pregnancy
1.1 Gestational Hypertension
- Definition and Diagnostic Criteria
- Gestational hypertension is defined as new-onset systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg documented on two occasions at least 4 hours apart that occurs at >20 weeks of gestation in a previously normotensive woman, without proteinuria or other signs of end-organ dysfunction at the time of diagnosis.
- Blood pressure elevations that first appear before 20 weeks of gestation should prompt evaluation for chronic hypertension rather than gestational hypertension.
- If hypertension develops after 20 weeks and proteinuria or end-organ dysfunction subsequently appears, the diagnosis is reclassified to preeclampsia.

- Epidemiology and Clinical Course
- Gestational hypertension may represent a transient hypertensive response to pregnancy in some women or may be a precursor to preeclampsia in others; approximately 15%–25% of women with gestational hypertension progress to preeclampsia.
- Onset is typically in the second half of pregnancy; resolution usually occurs by 6 weeks postpartum, but persistent hypertension beyond 12 weeks postpartum suggests chronic hypertension.
- Key Clinical Features
- Elevated blood pressure readings as described above, in the absence of proteinuria or systemic manifestations (e.g., thrombocytopenia, elevated liver enzymes, renal insufficiency, pulmonary edema, neurological symptoms).
- May be accompanied by mild peripheral edema, but generalized edema alone is not diagnostic.

- Differential Diagnosis
- Distinguish from chronic hypertension (preexisting or diagnosed before 20 weeks gestation), white coat hypertension, and masked hypertension.
- Confirm accurate measurement technique and consider ambulatory or home BP monitoring if readings are inconsistent.
- Nursing Insights
- Accurate blood pressure measurement technique is essential: use an appropriately sized cuff, ensure the patient is seated with back supported and legs uncrossed, arm at heart level, and allow 5 minutes of rest before measurement; document position and cuff size.
- Re-evaluate elevated readings with repeated measurements at least 4 hours apart; do not diagnose based on a single elevated value.
- Educate the patient on home blood pressure monitoring: instruct on frequency (e.g., twice daily), proper technique, and threshold values for reporting (report readings ≥140/90 mm Hg).
- Recognize gestational hypertension as a dynamic diagnosis that requires close follow-up because progression to preeclampsia may be sudden; establish a surveillance plan including BP checks and symptom review.
- Prepare patient education materials that explain warning signs (headache, visual changes, epigastric pain, oliguria, decreased fetal movement) and emergency contact instructions.
1.2 Preeclampsia
- Definition and Diagnostic Criteria
- Preeclampsia is a multisystem disorder of pregnancy defined by new-onset hypertension (systolic ≥140 mm Hg or diastolic ≥90 mm Hg on two occasions ≥4 hours apart) at >20 weeks gestation accompanied by either:
- Proteinuria defined as ≥300 mg protein in a 24-hour urine collection, protein/creatinine ratio ≥0.3, or dipstick reading ≥1+ when quantitative methods are unavailable; or
- In the absence of proteinuria, new-onset end-organ dysfunction including thrombocytopenia (platelets <100,000/µL), renal insufficiency (serum creatinine >1.1 mg/dL or doubling of baseline), impaired liver function (elevated transaminases), pulmonary edema, or new-onset cerebral or visual disturbances.
- Preeclampsia is a multisystem disorder of pregnancy defined by new-onset hypertension (systolic ≥140 mm Hg or diastolic ≥90 mm Hg on two occasions ≥4 hours apart) at >20 weeks gestation accompanied by either:
- Pathophysiologic Overview (brief, to orient classification)
- The central pathophysiologic process involves abnormal placentation with inadequate remodeling of the spiral arteries, resulting in placental hypoperfusion and ischemia; the ischemic placenta releases antiangiogenic factors (e.g., sFlt-1) and inflammatory mediators that provoke widespread maternal endothelial dysfunction, vasoconstriction, and a prothrombotic state.
- Clinical Spectrum
- Preeclampsia is a spectrum ranging from mild disease (preeclampsia without severe features) to severe disease (preeclampsia with severe features), and in its extreme form can lead to HELLP syndrome or eclampsia.
- Nursing Insights
- Assess for and document symptoms that indicate systemic involvement: persistent or severe headache, visual disturbances (scotomata, blurred vision), right upper quadrant or epigastric pain, sudden weight gain, and oliguria. These symptoms indicate progressive or severe disease and warrant urgent escalation.
- Monitor laboratory trends: serial platelet counts, AST/ALT, serum creatinine, and LDH when clinically indicated; create clear protocols for frequency of labs based on disease severity.
- Coordinate multidisciplinary care early (obstetrics, maternal-fetal medicine, anesthesia, critical care) when preeclampsia is diagnosed, as rapid deterioration is possible.
- Implement fetal surveillance appropriate to severity and gestational age: non-stress tests, biophysical profiles, and ultrasound growth assessments to detect fetal growth restriction and oligohydramnios.
- Ensure documentation includes baseline blood pressures, times of measurement, symptoms, urine protein quantification, and all nursing interventions.
1.2.1 Preeclampsia Without Severe Features
- Definition and Diagnostic Criteria
- Preeclampsia without severe features is characterized by new-onset hypertension (≥140/90 mm Hg) after 20 weeks of gestation plus proteinuria (≥300 mg/24 h or equivalent) or other diagnostic criteria for preeclampsia without evidence of severe features such as severe-range blood pressures, significant thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, or neurological symptoms.
- Clinical Presentation
- Patients typically have asymptomatic or mildly symptomatic disease: mild headache responsive to acetaminophen, mild peripheral edema, and modest proteinuria.
- Blood pressure generally in the range of 140–159 systolic or 90–109 diastolic.
- Monitoring and Management Principles
- Conservative management may be appropriate depending on gestational age and maternal/fetal status: outpatient management with frequent blood pressure checks, activity modification, serial laboratory assessment, and fetal surveillance can be considered for stable patients at or beyond a threshold gestational age.
- Consider antenatal corticosteroids if delivery is anticipated before 34 weeks and maternal/fetal status warrants it.
- Antihypertensive therapy is instituted when BP is persistently ≥150–160/100–110 mm Hg depending on institutional policy and maternal risk; thresholds vary but aim to prevent severe-range pressures.
- Nursing Insights
- For ambulatory patients, set up a structured follow-up schedule: BP checks (clinic or home), symptom diary (headache, vision changes, RUQ pain), and clear escalation criteria for urgent evaluation.
- Educate on activity and diet: no evidence supports strict bed rest; encourage moderate activity as tolerated and balanced nutrition; advise against high-sodium or fad diets.
- Teach accurate home urine protein testing only as adjunct; emphasize that quantitative testing (protein/creatinine ratio or 24-hour collection) is definitive.
- Recognize that even “without severe features” can rapidly evolve; instruct patients to present urgently for any concerning symptoms.
1.2.2 Preeclampsia With Severe Features
- Definition and Diagnostic Criteria
- Preeclampsia with severe features is defined as new-onset hypertension after 20 weeks gestation plus one or more of the following:
- Systolic blood pressure ≥160 mm Hg or diastolic ≥110 mm Hg on two occasions at least 4 hours apart (unless antihypertensive therapy is initiated), or
- Thrombocytopenia (platelets <100,000/µL), or
- Serum transaminases at least twice normal concentration, or
- Progressive renal insufficiency (serum creatinine >1.1 mg/dL or doubling of baseline), or
- Pulmonary edema, or
- New-onset cerebral or visual disturbances (e.g., severe headache, visual scotomata), or
- Persistent or severe epigastric or right upper quadrant pain.
- Preeclampsia with severe features is defined as new-onset hypertension after 20 weeks gestation plus one or more of the following:

- Clinical Implications
- Severe features signal significant end-organ involvement and substantially increase the risk of maternal morbidity (e.g., intracranial hemorrhage, pulmonary edema, hepatic rupture, DIC) and fetal compromise (IUGR, oligohydramnios, preterm birth).
- Immediate medical management and frequently expedited delivery are often indicated, depending on gestational age and stability.
- Management Priorities
- Stabilize maternal airway, breathing, and circulation; treat severe-range hypertension with rapid-acting antihypertensives (e.g., IV labetalol, IV hydralazine, or oral/NG nifedipine per protocol) to reduce risk of stroke.
- Initiate magnesium sulfate for seizure prophylaxis when indicated (generally for severe features) and continue per protocol post-delivery for a recommended period.
- Prepare for delivery when maternal or fetal condition indicates; coordinate anesthesia consult early because coagulopathy and thrombocytopenia may affect neuraxial anesthesia decisions.
- Nursing Insights
- Prioritize frequent vital signs and continuous maternal-fetal monitoring; document BP every 15 minutes during acute management, then at intervals per protocol.
- Perform and document neurological assessments frequently: level of consciousness, headache severity, visual changes, deep tendon reflexes, and presence of clonus. Escalate care immediately for signs of neurological deterioration.
- Monitor intake and output hourly and report oliguria (<30 mL/h) as this may indicate renal compromise or magnesium toxicity in the setting of magnesium therapy.
- Prepare blood products and have transfusion protocols ready if HELLP or DIC is suspected; obtain baseline coagulation studies and crossmatch as ordered.
- Ensure bedrest in a lateral position when feasible to enhance uteroplacental perfusion; implement seizure precautions including padded side rails and suction at bedside.
1.3 Eclampsia
- Definition
- Eclampsia is the occurrence of new-onset generalized tonic-clonic seizures or coma in a woman with preeclampsia that cannot be attributed to other neurologic conditions (e.g., epilepsy, cerebral hemorrhage, intoxication).
- Epidemiology and Clinical Course
- Eclampsia is less common in high-resource settings due to prophylactic use of magnesium sulfate and improved surveillance, but it remains a major contributor to maternal mortality in regions with limited access to care.
- Seizures may occur antepartum, intrapartum, or postpartum; up to one-third of eclamptic seizures occur postpartum.
- Pathophysiology
- Seizures in eclampsia are believed to result from severe cerebral vasospasm, endothelial disruption, and vasogenic cerebral edema leading to decreased seizure threshold and focal or generalized neuronal hyperexcitability.
- Clinical Features
- Prodromal symptoms may include severe headache, visual disturbances, agitation, or altered mental status, but seizures can also be abrupt and without warning.
- Nursing Insights
- During a seizure, the immediate nursing priorities are airway protection, maternal safety, and prevention of aspiration: position on the side once feasible, clear airway, do not place objects in mouth, and ensure oxygen and suction are available.
- Administer magnesium sulfate as first-line anticonvulsant therapy per protocol; have calcium gluconate available as the antidote for magnesium toxicity.
- After the seizure, perform a rapid assessment for injury, vaginal bleeding/placental abruption, cervical status, and fetal distress; continuous fetal monitoring is essential.
- Communicate rapidly with the multidisciplinary team to prepare for potential urgent delivery, intubation, imaging (CT/MRI if intracranial hemorrhage suspected), and critical care transfer.
1.4 Chronic Hypertension
- Definition
- Chronic hypertension is hypertension diagnosed prior to pregnancy or detected before 20 weeks of gestation; it includes women with preexisting essential hypertension and those with secondary causes of hypertension.
- Clinical Considerations
- Women with chronic hypertension are at increased risk of superimposed preeclampsia, fetal growth restriction, preterm birth, and placental abruption.
- Baseline antihypertensive therapies may require adjustment because certain antihypertensive agents (e.g., ACE inhibitors, ARBs) are contraindicated in pregnancy.
- Management Principles
- Optimize blood pressure control prior to conception when possible; during pregnancy, use pregnancy-safe antihypertensives (e.g., labetalol, nifedipine, methyldopa) and monitor for signs of superimposed preeclampsia.
- Monitor fetal growth and placental function with serial ultrasound and consider increased antenatal surveillance.
- Nursing Insights
- Obtain a thorough preconception/antepartum medication history and reconcile medications; educate patients about teratogenic drugs and the need for changes before or immediately after conception.
- Monitor for signs of superimposed preeclampsia: new or worsening proteinuria, sudden increase in BP, development of severe features—escalate care promptly.
- Educate on lifestyle modifications to support BP control: sodium moderation, weight management, smoking cessation, and adherence to prescribed medications.
- Coordinate care with primary care and cardiology when appropriate for complex or secondary hypertension.
1.5 Superimposed Preeclampsia
- Definition
- Superimposed preeclampsia occurs when a woman with chronic hypertension develops new-onset proteinuria or sudden worsening of hypertension with features consistent with preeclampsia after 20 weeks gestation, or when preexisting hypertension becomes accompanied by end-organ dysfunction typical of preeclampsia.
- Diagnostic Criteria
- New-onset proteinuria in a woman with chronic hypertension, or a sudden and sustained increase in blood pressure or development of severe features (thrombocytopenia, elevated transaminases, renal insufficiency, pulmonary edema, neurological symptoms) that cannot be explained by chronic hypertension alone.
- Clinical Importance
- Superimposed preeclampsia carries a higher risk of adverse maternal and fetal outcomes compared with preeclampsia or chronic hypertension alone, including higher rates of HELLP syndrome, placental abruption, and preterm delivery.
- Management Considerations
- Management is similar to preeclampsia with severe features when severe features are present; more intense surveillance is required including frequent laboratory monitoring and fetal assessment.
- Antihypertensive regimens may need to be intensified and magnesium sulfate initiated if severe features develop.
- Nursing Insights
- Maintain a high index of suspicion in women with chronic hypertension for new proteinuria or abrupt changes in BP; institute prompt urine protein testing and laboratory evaluation when indicated.
- Establish baseline laboratory values early in pregnancy for women with chronic hypertension to aid in detection of superimposed disease.
- Provide anticipatory guidance regarding the increased risk of adverse outcomes and the potential need for earlier delivery; discuss implications for anesthesia and possible blood product availability.
- Educate women and family about the signs of deterioration and ensure rapid access to care; arrange closer follow-up schedules.
Pathophysiology Of Hypertensive Disorders
Hypertensive disorders of pregnancy (HDP), particularly preeclampsia and eclampsia, arise from a complex interplay of placental, vascular, immunologic, and genetic factors. The underlying event is abnormal placentation that leads to endothelial dysfunction, vasospasm, and multi-organ microangiopathy. These processes compromise maternal perfusion and cause characteristic signs such as hypertension, proteinuria, and systemic involvement.
1.1 Abnormal Trophoblastic Invasion
In normal pregnancy, the cytotrophoblasts invade the maternal spiral arteries during early gestation (8–18 weeks). This physiologic remodeling transforms the small, high-resistance vessels into large, low-resistance channels to ensure adequate uteroplacental blood flow.
In preeclampsia:
- Trophoblastic invasion is incomplete.
- Spiral arteries remain narrow, muscular, and responsive to vasomotor stimuli.
- This results in reduced uteroplacental perfusion and relative placental ischemia.
Key cellular events:
- Impaired differentiation of endovascular cytotrophoblasts prevents normal vascular remodeling.
- The placental bed shows fibrinoid necrosis, atherosis, and inflammatory infiltration.
- The ischemic placenta releases antiangiogenic factors such as sFlt-1 and endoglin, which antagonize VEGF (vascular endothelial growth factor) and placental growth factor (PlGF).
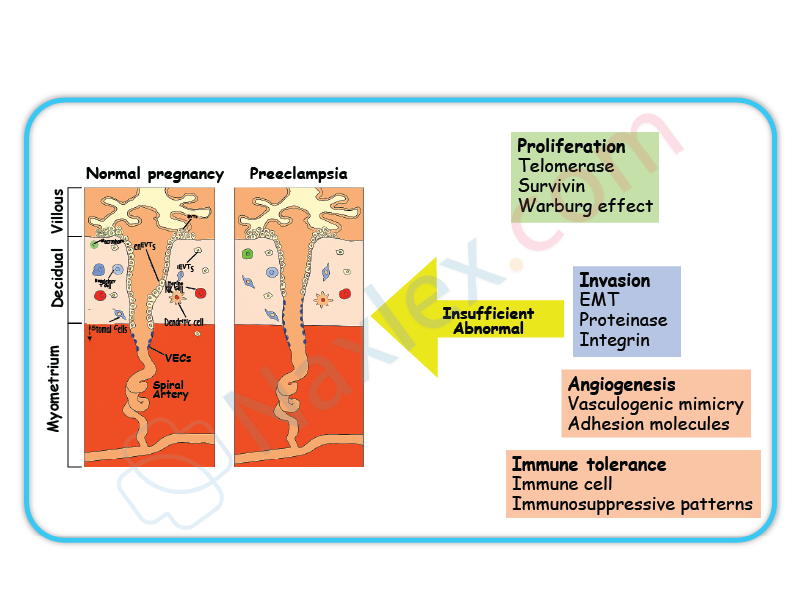
Pathophysiologic consequence:
Placental hypoxia → oxidative stress → systemic endothelial activation → vasoconstriction → hypertension.
Nursing Insight:
Early recognition of abnormal placental perfusion (via Doppler studies showing reduced diastolic flow) predicts the risk of preeclampsia. Nursing interventions should focus on monitoring fetal growth restriction and maternal symptoms early in high-risk pregnancies.
1.2 Endothelial Dysfunction
Endothelial cells normally produce vasodilators such as nitric oxide (NO) and prostacyclin (PGI₂), maintaining vascular tone and preventing platelet aggregation. In preeclampsia, endothelial injury shifts this balance toward vasoconstriction and coagulation.
Mechanisms:
- ↓ Nitric oxide and prostacyclin → vasoconstriction.
- ↑ Endothelin and thromboxane A₂ → increased vascular resistance.
- ↑ Capillary permeability → plasma leakage → edema and hemoconcentration.
- Activation of platelets and coagulation cascade → microthrombi formation.
|
Normal Endothelium |
Endothelial Dysfunction in Preeclampsia |
|
Balanced vasodilators (NO, PGI₂) |
Decreased NO and PGI₂ |
|
Minimal vascular reactivity |
Increased vascular sensitivity to angiotensin II |
|
Low platelet activation |
Platelet aggregation and thrombocytopenia |
|
Stable oncotic pressure |
Capillary leak → edema, proteinuria |
Clinical correlation:
- Elevated BP due to systemic vasoconstriction.
- Proteinuria from glomerular endotheliosis.
- Hemoconcentration and edema due to plasma leakage.
Nursing Insight:
Monitor blood pressure at consistent intervals, use appropriate cuff size, and assess for dependent and facial edema. Persistent headache and visual disturbances reflect endothelial dysfunction affecting cerebral circulation.
1.3 Vasospasm and Multisystem Involvement
The hallmark of preeclampsia is widespread vasospasm, which leads to ischemia and hypoperfusion of major organs.
Renal System
- Glomerular endotheliosis causes reduced glomerular filtration rate (GFR).
- Proteinuria ≥ 300 mg/24h results from leaky glomerular capillaries.
- Sodium and water retention → edema and increased BP.
Hepatic System
- Hepatic vasospasm leads to periportal necrosis and subcapsular hematoma.
- Elevated AST and ALT, right upper quadrant or epigastric pain.
- May progress to HELLP Syndrome (Hemolysis, Elevated Liver Enzymes, Low Platelet count).
Cerebral and Visual System
- Vasospasm causes cerebral edema and ischemia → headaches, hyperreflexia, visual disturbances, seizures (eclampsia).
- Retinal arteriolar spasm causes blurred vision and scotoma.
Cardiovascular System
- Increased systemic vascular resistance.
- Reduced plasma volume despite edema (third spacing).
- Hemoconcentration increases risk of thrombosis.
|
Organ System |
Effect of Vasospasm |
Clinical Manifestation |
|
Kidneys |
↓ GFR, proteinuria |
Oliguria, elevated creatinine |
|
Liver |
Ischemia, necrosis |
Epigastric pain, ↑ liver enzymes |
|
Brain |
Edema, ischemia |
Headache, seizures |
|
Eyes |
Retinal spasm |
Blurred vision |
|
Placenta |
Hypoperfusion |
IUGR, fetal distress |
Nursing Insight:
Monitor urine output (≥30 mL/hr), deep tendon reflexes, and neurologic signs. Deterioration suggests progression toward severe preeclampsia or impending eclampsia.
1.4 Coagulation Abnormalities
Endothelial injury exposes subendothelial collagen, triggering platelet aggregation and activation of the coagulation cascade.
Pathophysiologic outcomes:
- Platelet consumption → thrombocytopenia (<100,000/μL).
- Fibrin deposition in small vessels → microangiopathic hemolysis.
- Excessive activation may evolve into disseminated intravascular coagulation (DIC).
Clinical Indicators:
- Petechiae, bleeding gums, hematuria.
- Prolonged PT, aPTT, and decreased fibrinogen.
- Elevated D-dimers indicating fibrinolysis.
Nursing Insight:
Monitor platelet counts and coagulation profiles regularly. Avoid invasive procedures (e.g., IM injections) if platelet count is <100,000/μL.
1.5 Summary of Pathophysiologic Cascade
- Abnormal placentation → placental ischemia.
- Placenta releases antiangiogenic factors → endothelial injury.
- Endothelial dysfunction → vasospasm and capillary leak.
- Vasospasm → organ hypoperfusion and hypertension.
- Coagulation activation → thrombocytopenia and hemolysis.
- Resulting clinical picture → hypertension, proteinuria, edema, and multi-organ involvement.
Overall Conceptual Map:
Abnormal Placentation
↓
Placental Ischemia
↓
Endothelial Dysfunction
↓
Systemic Vasospasm
↓
↓ Organ Perfusion → Hypertension + Proteinuria + Multi-Organ Damage
Nursing Insight:
Pathophysiologic understanding guides timely nursing interventions — maintaining strict fluid balance, monitoring BP, observing for neurologic changes, and preventing seizure and renal complications.
Clinical Manifestations And Diagnostic Findings
1.1 Clinical Manifestations
Hypertensive disorders of pregnancy—gestational hypertension, preeclampsia, eclampsia, and HELLP syndrome—present a spectrum of clinical findings that evolve with the severity of endothelial dysfunction, vasospasm, and multiorgan involvement.
Key Clinical Manifestations:
- Elevated Blood Pressure
- Defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg on two occasions ≥4 hours apart after 20 weeks gestation in a previously normotensive woman.
- Severe features: Systolic ≥160 mmHg or diastolic ≥110 mmHg.
- Generalized Edema
- Pathological edema due to increased capillary permeability and decreased colloid osmotic pressure.
- Pitting edema, especially in the face, hands, and lower extremities.
- Proteinuria
- A hallmark of preeclampsia due to glomerular endotheliosis and increased glomerular permeability.
- Neurological Symptoms
- Headache (persistent, severe, frontal or occipital).
- Visual disturbances: blurred vision, scotomata, photophobia.
- Hyperreflexia and clonus due to CNS irritability.
- Gastrointestinal and Hepatic Symptoms
- Epigastric or right upper quadrant pain (due to hepatic capsule distention or subcapsular hematoma).
- Nausea and vomiting from hepatic involvement.
- Renal Manifestations
- Oliguria (<500 mL/24h) from renal vasospasm and reduced perfusion.
- Elevated serum creatinine and uric acid levels.
- Respiratory Manifestations
- Dyspnea from pulmonary edema due to capillary leak and left ventricular dysfunction.
- Hematologic Manifestations
- Thrombocytopenia and hemolysis (especially in HELLP syndrome).
- Fetal Manifestations
- Decreased uteroplacental perfusion leading to intrauterine growth restriction (IUGR) or fetal hypoxia.
Nursing Insights
- Persistent headache, visual disturbances, or epigastric pain are ominous warning signs of disease progression.
- Always assess deep tendon reflexes and clonus—increased neuromuscular irritability indicates risk of seizure onset.
- Report decreased fetal movement, which may indicate uteroplacental insufficiency.
1.2 Blood Pressure Criteria and Diagnostic Thresholds
|
Category |
Systolic (mmHg) |
Diastolic (mmHg) |
Timing |
Proteinuria |
Other Features |
|
Chronic Hypertension |
≥140 |
≥90 |
Before 20 weeks |
None |
|
|
Gestational Hypertension |
≥140 |
≥90 |
After 20 weeks |
None |
BP normalizes postpartum |
|
Preeclampsia (without severe features) |
≥140 but <160 |
≥90 but <110 |
After 20 weeks |
≥300 mg/24h or Protein/Creatinine ≥0.3 |
No organ dysfunction |
|
Preeclampsia with severe features |
≥160 |
≥110 |
After 20 weeks |
May or may not be present |
Evidence of organ damage |
|
Eclampsia |
Variable |
Variable |
After 20 weeks or postpartum |
Variable |
Seizures not due to other causes |
|
HELLP Syndrome |
Variable |
Variable |
Any time (usually 27–37 wks) |
Variable |
Hemolysis, Elevated Liver enzymes, Low Platelets |
Interpretation:
- Two readings, ≥4 hours apart, are required for diagnosis.
- Severe hypertension (≥160/110 mmHg) warrants immediate intervention to prevent maternal stroke.
Nursing Insights
- Always use the right cuff size; small cuffs overestimate BP.
- BP should be taken with the patient seated or in left lateral recumbent to avoid supine hypotension.
- Do not rely on a single BP reading for diagnosis; repeated accurate measurements are mandatory.
1.3 Proteinuria and Renal Assessment
Proteinuria Criteria:
- ≥300 mg protein in a 24-hour urine collection.
- Protein/creatinine ratio ≥0.3 mg/dL.
- Dipstick reading ≥1+ (if quantitative tests unavailable).
Renal Function Changes:
- Glomerular endotheliosis causes narrowing of capillary lumens and reduced filtration surface area.
- Serum Creatinine >1.1 mg/dL or doubling of baseline indicates renal insufficiency.
- Uric acid levels >5.5 mg/dL indicate decreased renal clearance.
- Oliguria (<500 mL/24h) is a sign of renal compromise.
|
Test |
Normal Pregnancy |
Preeclampsia |
|
Serum Creatinine |
0.4–0.8 mg/dL |
>1.1 mg/dL |
|
Uric Acid |
<5.5 mg/dL |
>6 mg/dL |
|
Proteinuria |
<150 mg/24h |
≥300 mg/24h |
Nursing Insights
- Monitor urine output hourly if magnesium sulfate is administered; decreased output increases toxicity risk.
- Collect 24-hour urine samples accurately—discard the first specimen, include all subsequent voids.
- Oliguria with dark urine suggests renal ischemia; report immediately.
1.4 Laboratory Tests (Liver, Platelet, Hemolysis Indices)
Key Laboratory Parameters:
- Platelet Count: <100,000/μL indicates thrombocytopenia.
- AST/ALT: Elevated >2× normal suggests hepatic injury.
- LDH (>600 IU/L): Indicates hemolysis.
- Peripheral Smear: Schistocytes confirm microangiopathic hemolysis.
- Haptoglobin: Decreased levels support diagnosis of hemolysis.
- Bilirubin: Mild elevation due to RBC breakdown.
- Serum Creatinine & Uric Acid: Elevated in renal involvement.
|
Test |
Normal Range |
Abnormal in HELLP |
Clinical Significance |
|
Platelets |
150,000–400,000 |
<100,000 |
Risk of bleeding |
|
AST/ALT |
<40 IU/L |
>70 IU/L |
Hepatic damage |
|
LDH |
<200 IU/L |
>600 IU/L |
Hemolysis |
|
Haptoglobin |
30–200 mg/dL |
Decreased |
Hemolysis marker |
Nursing Insights
- Always check trends, not single values—falling platelet count indicates disease progression.
- Avoid IM injections in thrombocytopenic patients to prevent hematoma.
- Right upper quadrant pain + elevated AST/ALT = impending hepatic rupture.
1.5 Fetal Assessment Findings
Fetal Complications:
- Intrauterine growth restriction (IUGR)
- Oligohydramnios
- Fetal distress
- Placental abruption
- Preterm delivery
- Intrauterine fetal demise (in severe cases)
Assessment Methods:
- Non-Stress Test (NST): Monitors fetal heart rate reactivity.
- Biophysical Profile (BPP): Evaluates fetal breathing, movement, tone, amniotic fluid volume, and heart rate.
- Doppler Velocimetry: Assesses umbilical artery blood flow resistance.
- Ultrasound: Monitors fetal growth and amniotic fluid index.
|
Test |
Normal Result |
Abnormal Finding |
Interpretation |
|
NST |
Reactive (≥2 accelerations/20 min) |
Non-reactive |
Fetal hypoxia |
|
BPP |
8–10 |
≤6 |
Compromised fetus |
|
Doppler S/D ratio |
<3.0 |
>3.0 |
Increased placental resistance |
Nursing Insights
- Daily fetal movement counts are vital for self-monitoring.
- If NST is non-reactive, prepare for BPP or CST (Contraction Stress Test).
Severe preeclampsia with non-reassuring fetal testing = indication for delivery.
Management Of Hypertensive Disorders In Pregnancy
Hypertensive disorders in pregnancy (HDP) encompass a spectrum of conditions including gestational hypertension, preeclampsia, eclampsia, chronic hypertension, and HELLP syndrome. The management focuses on maternal stabilization, prevention of complications (seizures, organ failure, stroke), and ensuring optimal fetal outcomes. Interventions are tailored based on disease severity, gestational age, and maternal-fetal condition.
1.1 Nursing Assessment and Monitoring
Accurate and continuous assessment is critical in managing hypertensive disorders in pregnancy to detect early deterioration and prevent maternal-fetal morbidity.
Assessment Components
- Blood Pressure (BP) Monitoring
- Measure BP using the same arm, in sitting or left lateral recumbent position.
- Use appropriately sized cuff; incorrect cuff size may falsely elevate readings.
- Diagnostic threshold:
- ≥140/90 mmHg after 20 weeks → Hypertensive disorder.
- ≥160/110 mmHg → Severe preeclampsia.
- Urinalysis
- Evaluate for proteinuria (≥300 mg/24 hr or +1 on dipstick).
- Protein/creatinine ratio ≥0.3 indicates significant proteinuria.
- Neurological Assessment
- Assess for headache, visual disturbances, hyperreflexia, clonus, confusion, or seizure activity.
- Respiratory Assessment
- Monitor for dyspnea, crackles, pulmonary edema.
- Renal Function
- Measure urine output (>30 mL/hr), serum creatinine, and BUN levels.
- Hepatic Function
- Monitor AST, ALT, and LDH for hepatic involvement or HELLP syndrome.
- Fetal Surveillance
- Conduct non-stress tests (NSTs), biophysical profiles (BPPs), and Doppler velocimetry.
- Assess for intrauterine growth restriction (IUGR).
Nursing Insights
- Frequent BP checks are vital; a sudden rise >30 mmHg systolic or >15 mmHg diastolic from baseline, even if <140/90 mmHg, can still signify pathology.
- Always assess deep tendon reflexes (DTRs)—hyperreflexia may indicate impending eclampsia.
- Monitor weight gain—>1 kg/week may indicate fluid retention.
- Document findings meticulously and report epigastric pain or visual changes immediately as these precede seizures.
1.2 Pharmacologic Management
Pharmacologic therapy aims to control hypertension, prevent seizures, and minimize maternal and fetal complications.
1.2.1 Antihypertensives
Commonly Used Medications
|
Medication |
Class |
Mechanism |
Key Nursing Considerations |
|
Labetalol |
β-blocker |
Reduces systemic vascular resistance without lowering cardiac output |
Monitor for bradycardia, avoid in asthma |
|
Hydralazine |
Vasodilator |
Direct arteriolar smooth muscle relaxation |
Monitor for reflex tachycardia, headache |
|
Nifedipine |
Calcium channel blocker |
Inhibits calcium ion influx in vascular smooth muscle |
Administer orally, avoid co-administration with MgSO₄ IV |
|
Methyldopa |
Centrally acting α2 agonist |
Reduces sympathetic tone |
Preferred for long-term control, monitor for sedation or liver dysfunction |
Therapeutic Goal
- Maintain systolic BP 130–150 mmHg and diastolic BP 80–100 mmHg.
- Avoid lowering BP too rapidly to prevent placental hypoperfusion.
Nursing Insights
- IV hydralazine or IV labetalol are used for acute severe hypertension.
- Avoid ACE inhibitors and ARBs—teratogenic and fetotoxic.
- Assess fetal heart rate (FHR) after maternal antihypertensive administration to ensure placental perfusion.
1.2.2 Magnesium Sulfate Therapy
Used for seizure prophylaxis and treatment in severe preeclampsia and eclampsia.
Mechanism of Action
- CNS depressant → reduces acetylcholine release at neuromuscular junction → prevents seizures.
- Also vasodilates cerebral vessels → reduces ischemia.
Dosage and Administration
- Loading dose: 4–6 g IV over 20–30 minutes.
- Maintenance: 2 g/hr continuous infusion.
- Continue for 24 hours postpartum or after last seizure.
Toxicity Monitoring
|
Parameter |
Normal |
Toxicity Indicator |
|
Deep tendon reflexes (DTRs) |
2+ |
Absent reflexes |
|
Respirations |
12-20/min |
<12/min |
|
Urine output |
≥30 mL/hr |
<30 mL/hr |
|
Serum Mg²⁺ |
4–7 mEq/L |
>8 mEq/L (toxic) |
Antidote
- Calcium gluconate 10%, 1 g (10 mL) IV over 3 minutes.
Nursing Insights
- Always use an infusion pump.
- Keep calcium gluconate readily available at bedside.
- Continuous fetal monitoring during therapy—maternal toxicity can cause fetal bradycardia.
- Report flushing, muscle weakness, or slurred speech promptly.
1.2.3 Corticosteroid Therapy
Administered for fetal lung maturation and maternal stabilization in HELLP syndrome.
Common Drugs
- Betamethasone 12 mg IM every 24 hours × 2 doses.
- Dexamethasone 6 mg IM every 12 hours × 4 doses.
Indications
- Gestational age <34 weeks with preeclampsia/eclampsia.
- HELLP syndrome to improve platelet count and hepatic function.
Nursing Insights
- Monitor maternal glucose—corticosteroids can cause transient hyperglycemia.
- Observe for signs of infection—steroids can suppress immune response.
1.3 Fluid Management
Fluid therapy in hypertensive disorders requires caution due to the risk of pulmonary edema and renal compromise.
Guidelines
- Restrict total fluid intake to ~80 mL/hr or <100 mL/hr.
- Maintain urine output ≥30 mL/hr.
- Avoid fluid boluses unless in hypovolemic shock.
- Use crystalloids; avoid colloids unless severe hypoproteinemia.
Monitoring
- Strict intake and output (I&O) charting.
- Daily weights and lung auscultation to detect early fluid overload.
- Evaluate central venous pressure (CVP) if indicated.
Nursing Insights
- Overhydration can precipitate pulmonary edema especially with MgSO₄ therapy.
- In HELLP syndrome, volume expansion may improve perfusion but must be closely monitored.
1.4 Nutritional and Lifestyle Modifications
- Dietary Management
- Balanced diet rich in protein, calcium, vitamin C, and fiber.
- Avoid excess sodium intake.
- Encourage adequate hydration, unless contraindicated.
- Activity
- Encourage bed rest in left lateral position to improve uteroplacental blood flow.
- Reduce stress and provide psychological support.
- Weight Monitoring
- Weekly weight monitoring to detect fluid retention.
Nursing Insights
- Educate patient on home BP monitoring and symptom tracking (headache, vision changes, epigastric pain).
- Encourage follow-up visits and postpartum surveillance for at least 6 weeks.
1.5 Timing and Mode of Delivery
Decision Factors
- Gestational age
- Severity of maternal disease
- Fetal condition and viability
|
Condition |
Recommended Timing |
Mode of Delivery |
|
Gestational hypertension |
≥37 weeks |
Vaginal preferred |
|
Preeclampsia without severe features |
37 weeks |
Vaginal induction |
|
Severe preeclampsia |
≥34 weeks or earlier if maternal/fetal distress |
Cesarean section may be indicated |
|
Eclampsia |
After stabilization and seizure control |
Expedite delivery—vaginal if feasible |
Nursing Insights
- Never induce labor in unstable eclamptic patients until airway and seizures are managed.
- Magnesium sulfate should be continued throughout labor and for 24 hours postpartum.
1.6 Postpartum Management
Postpartum care focuses on preventing complications, monitoring resolution, and educating for future risk reduction.
Key Interventions
- Continue BP monitoring for ≥72 hours after delivery.
- Maintain MgSO₄ infusion for 24 hours post-delivery or last seizure.
- Observe for:
- Seizures
- Pulmonary edema
- Renal failure
- Postpartum hemorrhage due to uterine atony from MgSO₄.
- Long-term counseling
- Risk of recurrence in future pregnancies.
- Increased lifetime risk of cardiovascular disease.
Nursing Insights
- Teach the mother about warning signs (severe headache, visual changes, swelling, chest pain).
- Advise follow-up for renal function and BP checks at 6 weeks postpartum.
- Encourage contraceptive counseling—avoid estrogen-containing contraceptives in persistent hypertension.
Hellp Syndrome (Hemolysis, Elevated Liver Enzymes, Low Platelet Count)
1.1 Definition and Diagnostic Criteria
- HELLP syndrome is a severe variant of preeclampsia characterized by:
- H: Hemolysis – destruction of red blood cells.
- EL: Elevated liver enzymes – indicating liver dysfunction.
- LP: Low platelet count – thrombocytopenia <100,000/μL.
- Diagnostic criteria typically include:
- Hemolysis: Presence of schistocytes on peripheral blood smear, elevated lactate dehydrogenase (LDH >600 IU/L), decreased haptoglobin.
- Elevated Liver Enzymes: AST or ALT >70 IU/L.
- Low Platelets: <100,000/μL.
- HELLP syndrome may present with or without hypertension or proteinuria.
Nursing Insights:
- Early recognition is critical; HELLP can progress rapidly and lead to life-threatening maternal and fetal complications.
- Blood pressure may not always be severely elevated; nurses must rely on laboratory values and symptoms.
- Educate staff to monitor patients with preeclampsia for sudden right upper quadrant pain, nausea, or malaise, which may indicate HELLP.
1.2 Pathophysiology
- Endothelial dysfunction leads to microvascular damage.
- Hemolysis occurs due to microangiopathic destruction of RBCs as they pass through narrowed vessels.
- Liver dysfunction is caused by periportal hemorrhage, fibrin deposition, and hepatocellular necrosis.
- Platelet consumption occurs in the formation of microthrombi, causing thrombocytopenia.
- Associated with abnormal placentation, vasospasm, and systemic inflammatory response.
Nursing Insights:
- Nurses must understand that HELLP is part of the preeclampsia spectrum, not a separate disease.
- Continuous monitoring of labs and maternal/fetal status is essential due to rapid deterioration risk.
1.3 Clinical Manifestations
- Common Symptoms:
- Right upper quadrant or epigastric pain.
- Nausea and vomiting.
- Malaise or fatigue.
- Headache and visual disturbances.
- Hypertension may be present but not always.
- Physical Findings:
- Jaundice (from hemolysis).
- Edema.
- Signs of bleeding (petechiae, ecchymosis).
Nursing Insights:
- Monitor for subtle signs of liver dysfunction, such as persistent epigastric pain unrelieved by antacids.
- Assess for bleeding tendencies due to low platelets.
- Educate patient to report sudden headaches, visual changes, or right upper quadrant pain immediately.
1.4 Laboratory Findings
|
Parameter |
Expected Finding in HELLP Syndrome |
Nursing Implications |
|
Hemolysis |
Schistocytes on smear, LDH >600 IU/L, low haptoglobin |
Monitor for anemia, tachycardia, pallor |
|
Liver Enzymes |
AST/ALT >70 IU/L |
Assess for hepatic tenderness, jaundice |
|
Platelets |
<100,000/μL |
Monitor for bleeding, avoid invasive procedures |
|
Bilirubin |
Mildly elevated |
Monitor for jaundice and hemolysis |
|
Urinalysis |
Variable proteinuria |
Assess renal function and risk of preeclampsia |
Nursing Insights:
- Laboratory trends are more important than a single value.
- Frequent monitoring is essential; rapid deterioration can occur within hours.
1.5 Management and Nursing Interventions
- Definitive Treatment: Delivery of the fetus; timing depends on gestational age and maternal/fetal stability.
- Medical Management Prior to Delivery:
- Blood pressure control (Labetalol, Hydralazine, Nifedipine).
- Seizure prophylaxis: Magnesium sulfate.
- Corticosteroids to enhance fetal lung maturity if preterm.
- Platelet transfusion if count <50,000/μL prior to cesarean or invasive procedures.
- Supportive Care:
- Bed rest in left lateral position.
- Monitor input/output, daily weights.
- Oxygen therapy if hypoxia.
- Monitor for complications: DIC, hepatic rupture, renal failure.
Nursing Insights:
- Continuous maternal and fetal monitoring is mandatory.
- Prepare for emergency delivery if signs of deterioration arise.
- Educate patient and family about the seriousness of HELLP syndrome and expected interventions.
- Monitor for magnesium sulfate toxicity: absent deep tendon reflexes, respiratory depression, decreased urine output.
1.6 Maternal and Fetal Complications
- Maternal:
- Disseminated intravascular coagulation (DIC).
- Hepatic rupture or subcapsular hematoma.
- Acute renal failure.
- Pulmonary edema.
- Placental abruption.
- Fetal:
- Preterm birth.
- Intrauterine growth restriction (IUGR).
- Fetal hypoxia.
- Stillbirth.
Nursing Insights:
- Anticipate NICU admission for preterm infants.
- Assess for signs of maternal shock or bleeding.
- Educate parents on potential neonatal complications and need for monitoring.
Eclampsia: Seizure Management And Nursing Care
1.1 Pathophysiology of Seizures
- Eclampsia is defined as the occurrence of tonic-clonic seizures in a woman with preeclampsia, not attributable to other neurological conditions.
- Seizures are caused primarily by cerebral vasospasm, endothelial dysfunction, and increased blood-brain barrier permeability, leading to cerebral edema.
- Cerebral vasospasm results in decreased cerebral perfusion, ischemia, and hypoxia, precipitating neuronal hyperexcitability and seizure activity.
- Endothelial injury triggers microvascular leakage, protein extravasation, and contributes to increased intracranial pressure.
- Magnesium sulfate is used as prophylaxis and treatment because it stabilizes neuronal membranes, acts as a CNS depressant, and reduces neuromuscular excitability.
Nursing Insights
- Nurses must understand that seizures in eclampsia are a medical emergency; rapid recognition and intervention are critical to prevent maternal and fetal morbidity.
- The pathophysiological cascade of vasospasm → edema → ischemia → seizure explains the need for blood pressure control and magnesium therapy.
- Seizure prophylaxis in preeclampsia with severe features reduces the risk of progression to eclampsia by 50–70%.
1.2 Clinical Manifestations
- Prodromal signs: Severe headache, visual disturbances (scotomata, blurred vision), hyperreflexia, epigastric or right upper quadrant pain.
- Seizure characteristics: Tonic-clonic movements, loss of consciousness, possible tongue biting, incontinence.
- Vital signs: Marked hypertension (systolic ≥160 mmHg, diastolic ≥110 mmHg), tachycardia, variable respiratory pattern.
- Laboratory abnormalities: May include hemolysis, elevated liver enzymes, low platelets (HELLP syndrome), and proteinuria.
Nursing Insights
- Early identification of prodromal signs allows for preemptive magnesium sulfate administration to prevent seizure occurrence.
- Monitoring for neurological changes is critical; nurses should document reflexes, level of consciousness, and visual disturbances.
- Maternal and fetal monitoring must be continuous, especially during seizure episodes, to detect hypoxia or fetal distress.
1.3 Nursing Interventions During a Seizure
- Immediate safety measures:
- Position patient on her side (left lateral preferred) to maintain airway and reduce aspiration risk.
- Remove sharp objects from the environment to prevent injury.
- Do not restrain the patient forcefully; allow movements while preventing harm.
- Airway management:
- Maintain patent airway; provide supplemental oxygen if needed.
- Prepare for suctioning if secretions compromise airway.
- Medication administration:
- Administer IV magnesium sulfate as ordered for seizure control.
- Antihypertensives (e.g., labetalol, hydralazine) to control severe hypertension.
- Monitoring:
- Continuous maternal vital signs and fetal heart rate monitoring.
- Assess for trauma, incontinence, and postictal confusion after seizure ends.
Nursing Insights
- Nurses should anticipate and prepare emergency equipment: oxygen, suction, IV access, magnesium sulfate, and antihypertensives.
- Protecting the patient from injury during seizure outweighs all other interventions initially.
- Understanding magnesium sulfate pharmacology (therapeutic range 4–7 mEq/L) allows early recognition of toxicity (loss of reflexes, respiratory depression).
1.4 Post-Seizure Care
- Assess airway, breathing, circulation immediately after seizure termination.
- Reposition patient to left lateral position to optimize placental perfusion.
- Monitor vital signs every 5–15 minutes until stable.
- Evaluate neurological status: level of consciousness, pupil reaction, reflexes.
- Check urine output to monitor renal perfusion and magnesium excretion.
- Assess fetal status: non-stress test, continuous electronic fetal monitoring.
- Document seizure duration, type, medications administered, and maternal/fetal response.
Nursing Insights
- Postictal monitoring is critical for secondary complications, including hypoxia, aspiration, and recurrence of seizure.
- Nurses should anticipate possible progression to HELLP syndrome or multi-organ dysfunction post-seizure.
- Early initiation of IV fluids with caution prevents pulmonary edema in hypertensive patients.
1.5 Prevention and Long-Term Management
- Seizure prophylaxis: IV magnesium sulfate for preeclampsia with severe features.
- Blood pressure control: Labetalol, hydralazine, nifedipine as indicated.
- Patient education: Recognize warning signs (headache, visual changes, epigastric pain), adhere to antihypertensive therapy, and report symptoms immediately.
- Postpartum monitoring: Blood pressure and neurological status for at least 72 hours postpartum; complications can occur after delivery.
- Long-term management: Counsel on future cardiovascular risks, including chronic hypertension and stroke risk.
Nursing Insights
- Nurses must emphasize early recognition and intervention for recurrent preeclampsia in future pregnancies.
- Continuous patient education is necessary for adherence to lifestyle modifications: low-sodium diet, regular prenatal visits, and blood pressure monitoring.
- Follow-up includes renal function and liver enzymes in patients with HELLP syndrome history.
Maternal And Fetal Complications Of Hypertensive Disorders
1.1 Maternal Complications
- Definition: Hypertensive disorders of pregnancy (HDP) encompass a spectrum of conditions including gestational hypertension, preeclampsia, eclampsia, and HELLP syndrome, all of which can significantly affect maternal physiology.
- Acute Maternal Complications:
- Cerebral Complications:
- Cerebral edema, vasospasm, and hemorrhage leading to seizures or stroke.
- Visual disturbances: scotomata, blurred vision, and retinal detachment in severe preeclampsia.
- Nursing Insights: Monitor neurological status continuously, assess deep tendon reflexes, and report sudden changes such as severe headache or visual blurring.
- Cardiovascular Complications:
- Pulmonary edema due to increased vascular permeability and left ventricular dysfunction.
- Severe hypertension (>160/110 mmHg) can precipitate myocardial ischemia.
- Nursing Insights: Assess lung sounds, oxygen saturation, and maintain strict fluid balance. Administer antihypertensives as ordered.
- Renal Complications:
- Acute kidney injury due to glomerular endotheliosis.
- Proteinuria ≥300 mg/24h, elevated serum creatinine.
- Nursing Insights: Monitor urine output hourly, daily weights, and renal laboratory parameters.
- Hepatic Complications:
- HELLP syndrome: hemolysis, elevated liver enzymes (AST, ALT), low platelets (<100,000/mm³).
- Risk of hepatic rupture or subcapsular hematoma.
- Nursing Insights: Monitor right upper quadrant pain, liver function tests, and prepare for emergent interventions.
- Hematologic Complications:
- Thrombocytopenia increases risk of hemorrhage during labor or postpartum.
- Disseminated intravascular coagulation (DIC) can develop.
- Nursing Insights: Monitor CBC, coagulation profiles, and implement bleeding precautions.
- Cerebral Complications:
- Long-Term Maternal Complications:
- Increased risk of chronic hypertension, cardiovascular disease, and renal disease later in life.
- Nursing Insights: Educate patients about lifestyle modification, follow-up blood pressure monitoring, and cardiovascular risk reduction.
1.2 Fetal and Neonatal Complications
- Placental Insufficiency:
- Inadequate uteroplacental perfusion leads to intrauterine growth restriction (IUGR).
- Nursing Insights: Monitor fetal growth via serial ultrasounds and Doppler studies.
- Prematurity:
- Early delivery may be required for maternal/fetal indications, increasing risk for neonatal morbidity.
- Nursing Insights: Administer corticosteroids (e.g., Betamethasone) to enhance fetal lung maturity when preterm birth is anticipated.
- Hypoxia and Acidosis:
- Chronic uteroplacental insufficiency may lead to fetal hypoxemia and acidemia.
- Nursing Insights: Continuous fetal monitoring, non-stress tests, and biophysical profiles are critical.
- Perinatal Mortality:
- Severe preeclampsia/eclampsia increases the risk of stillbirth and neonatal death.
- Nursing Insights: Ensure timely delivery and neonatal resuscitation preparedness.
- Long-Term Neonatal Sequelae:
- Increased risk for neurodevelopmental delays and cardiovascular issues in offspring.
1.3 Long-Term Sequelae for Mother and Child
- Maternal:
- Persisting hypertension, increased risk of ischemic heart disease, stroke, and chronic kidney disease.
- Nursing Insights: Encourage cardiovascular risk assessment, lifestyle modification, and regular follow-up.
- Child:
- Offspring may have low birth weight, developmental delays, and increased susceptibility to hypertension and metabolic syndrome later in life.
- Nursing Insights: Monitor growth and developmental milestones, coordinate pediatric follow-up care.
Summary
Definition and Classification of Hypertensive Disorders in Pregnancy
- Gestational Hypertension
- Blood pressure ≥140/90 mmHg after 20 weeks of gestation without proteinuria.
- No previous history of hypertension prior to pregnancy.
- Usually resolves by 12 weeks postpartum.
- Nursing Insights: Monitor for progression to preeclampsia, teach self-monitoring of blood pressure at home, and encourage rest and lifestyle modifications.
- Preeclampsia
- Blood pressure ≥140/90 mmHg after 20 weeks gestation with proteinuria (≥300 mg/24 hours) or signs of end-organ dysfunction.
- Classified into:
- Without severe features: BP 140–159/90–109 mmHg, mild proteinuria, absence of end-organ damage.
- With severe features: BP ≥160/110 mmHg, severe proteinuria, neurological symptoms (headache, visual changes), epigastric or right upper quadrant pain, pulmonary edema, renal or hepatic dysfunction.
- Nursing Insights: Frequent maternal and fetal monitoring is essential, educate patient about warning signs (headache, visual disturbances, epigastric pain), prepare for potential early delivery.
- Eclampsia
- Onset of seizures in a woman with preeclampsia not attributable to other causes.
- Seizures may occur antepartum, intrapartum, or postpartum.
- Nursing Insights: Priority interventions include airway protection, seizure management with magnesium sulfate, continuous fetal monitoring, and rapid response to maternal complications.
- Chronic Hypertension
- Hypertension present before pregnancy or diagnosed before 20 weeks gestation.
- May coexist with superimposed preeclampsia.
- Nursing Insights: Monitor for maternal complications (stroke, renal failure) and fetal complications (IUGR, preterm birth).
- HELLP Syndrome (Hemolysis, Elevated Liver enzymes, Low Platelets)
- A severe variant of preeclampsia.
- Lab findings:
- Hemolysis: schistocytes, elevated LDH
- Elevated liver enzymes: AST, ALT
- Low platelets: <100,000/μL
- Clinical manifestations: epigastric or right upper quadrant pain, nausea, malaise, possible jaundice.
- Nursing Insights: Monitor for signs of bleeding, prepare for transfusion or early delivery, frequent lab monitoring, assess for liver rupture or DIC.
Pathophysiology
- Abnormal trophoblastic invasion leads to inadequate remodeling of spiral arteries, resulting in poor uteroplacental perfusion.
- Endothelial dysfunction: causes vasoconstriction, platelet aggregation, increased vascular permeability, and end-organ ischemia.
- Anti-angiogenic factors like sFlt-1 inhibit VEGF and placental growth factor, contributing to hypertension and proteinuria.
Clinical Manifestations
|
Feature |
Preeclampsia Without Severe Features |
Preeclampsia With Severe Features |
HELLP Syndrome |
|
BP |
140–159/90–109 mmHg |
≥160/110 mmHg |
Often ≥160/110 mmHg |
|
Proteinuria |
≥300 mg/24 hr |
Severe |
Variable |
|
Edema |
Mild |
Generalized |
Often present |
|
Neurological Symptoms |
Rare |
Headache, visual disturbances |
May occur |
|
GI Symptoms |
Nausea mild |
Epigastric pain |
Severe epigastric/RUQ pain, N/V |
|
Lab Abnormalities |
Mild |
Elevated creatinine, LFTs |
Hemolysis, elevated AST/ALT, thrombocytopenia |
Complications
- Maternal: cerebral hemorrhage, pulmonary edema, renal failure, abruptio placentae, DIC.
- Fetal: IUGR, preterm birth, hypoxia, stillbirth.
Management Principles
- Delivery is the definitive treatment for preeclampsia and HELLP syndrome.
- Seizure prophylaxis: Magnesium sulfate infusion.
- Monitor for toxicity: respiratory rate <12, loss of deep tendon reflexes, decreased urine output.
- Antidote: calcium gluconate.
- Blood pressure control: Labetalol, hydralazine, nifedipine.
- Corticosteroids: Betamethasone for fetal lung maturity if preterm delivery anticipated.
Postpartum Considerations
- Hypertensive disorders may persist or present up to 12 weeks postpartum.
- Monitor BP, urine output, neurological status.
- Patient education on warning signs: headaches, visual changes, epigastric pain, and swelling.
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Hypertensive Disorders
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


