Please set your exam date
Drugs Used to Treat Heart Failure
Study Questions
Practice Questions 1
Scenario
An elderly client with known heart failure, who has previously been stable with lisinopril and metoprolol XL, comes into the clinic with an increase in weight and peripheral edema extending halfway up the left calf.
Before treatment is started, the nurse gathers assessment data for the client and recognizes which of the following symptoms are related to this condition? Select all that apply
Explanation
Heart failure is a complex clinical syndrome resulting from structural or functional myocardial impairment that compromises ventricular filling or the ejection of blood. It triggers chronic neurohormonal activation and systemic venous congestion, manifesting as dyspnea, fatigue, and fluid retention. Patients often present with elevated B-type natriuretic peptide levels ≥ 100 pg/mL and reduced cardiac output.
Rationale for correct answers
A. Impaired cardiac output and poor skeletal muscle perfusion lead to significant exercise intolerance and exertional dyspnea in failure patients. The heart cannot meet the increased metabolic demands of physical activity, resulting in rapid onset of fatigue. This symptom is a hallmark of both systolic and diastolic ventricular dysfunction. It represents the functional decline associated with reduced hemodynamic reserve.
C. Increased hydrostatic pressure in the systemic venous circulation causes fluid extravasation into the interstitium, appearing as dependent edema around the ankles. This peripheral swelling is a primary sign of right-sided heart failure and fluid volume excess. It often worsens throughout the day as gravity promotes fluid accumulation in the lower extremities. Monitoring for pitting edema is a critical nursing assessment for volume status.
D. Systemic venous congestion extends to the gastrointestinal tract, causing vascular engorgement of the stomach and intestines that results in anorexia and nausea. This congestion impairs normal digestion and leads to a feeling of fullness or abdominal discomfort. These symptoms can contribute to cardiac cachexia in advanced stages of the disease. The nurse must assess nutritional intake as an indicator of visceral congestion severity.
E. Right-sided heart failure causes backward pressure into the hepatic veins, leading to venous congestion of the liver, known as hepatomegaly or liver enlargement. The liver may become tender to palpation, and chronic congestion can eventually lead to cardiac cirrhosis or impaired hepatic function. This finding confirms the presence of systemic venous hypertension and right ventricular dysfunction. It is a classic physical sign of advanced congestive failure.
H. Reduced stroke volume and compensatory peripheral vasoconstriction result in poor perfusion to the skin and skeletal muscles. This manifests clinically as cool extremities, delayed capillary refill, and peripheral cyanosis in severe cases of low-output failure. The body shunts blood to vital organs, sacrificing blood flow to the distal tissues and skin. Assessing skin temperature and color provides immediate data on the adequacy of cardiac output.
Rationale for incorrect answers
B. Heart failure typically triggers a compensatory sympathetic nervous system response, resulting in tachycardia rather than bradycardia to maintain cardiac output. A slow heart rate is usually an adverse effect of medications like beta blockers or digitalis rather than a primary symptom of the disease. Tachycardia helps compensate for a decreased stroke volume according to the CO = HR x SV equation. Bradycardia would further compromise an already failing heart.
F. Clients with heart failure often exhibit a narrowed rather than a widening pulse pressure due to a significant reduction in stroke volume. A wide pulse pressure is more characteristic of conditions like aortic regurgitation or severe atherosclerosis in the elderly. In heart failure, the systolic pressure drops while diastolic pressure may rise due to vasoconstriction. This narrowing reflects the heart's inability to eject blood forcefully into the aorta.
G. While the liver is frequently enlarged due to its direct venous connection via the hepatic veins, splenomegaly is not a common or standard finding in heart failure. Spleen enlargement is more typically associated with portal hypertension from primary liver disease or hematological malignancies. Although severe right-sided failure can cause some splenic congestion, it does not routinely present as palpable splenomegaly. Hepatomegaly remains the more reliable indicator of systemic venous backup.
Test-taking strategy
- Identify the pathophysiology of heart failure: Differentiate between "forward" failure (low output/perfusion) and "backward" failure (congestion).
- Categorize symptoms by heart side:
- Left-sided: Pulmonary congestion (dyspnea, crackles).
- Right-sided: Systemic congestion (edema, hepatomegaly, GI symptoms).
- Use the concept of compensation: Recall that the body activates the sympathetic nervous system to increase heart rate, making tachycardia the expected finding, not bradycardia (Choice 2).
- Analyze perfusion and volume:
- Choice 1 and 8 relate to low output/poor perfusion.
- Choice 3, 4, and 5 relate to venous congestion/volume excess.
- Rule out unrelated clinical signs:
- Choice 6 (widening pulse pressure) is a classic distractor; heart failure narrows pulse pressure.
- Choice 7 (splenomegaly) is less specific than hepatomegaly for cardiac issues.
- Select comprehensive answers: In a "select all that apply" question, ensure all aspects of the disease (both forward and backward failure) are represented in the final selection.
Take home points
- Heart failure symptoms are classified into left-sided pulmonary congestion and right-sided systemic venous congestion including hepatomegaly and peripheral edema.
- Anorexia and nausea in heart failure are caused by venous congestion of the gastrointestinal tract, which can lead to significant nutritional deficiencies.
- Poor peripheral perfusion and exercise intolerance result from the heart's inability to maintain an adequate cardiac output to meet metabolic tissue demands.
- Tachycardia is a primary compensatory mechanism in heart failure, whereas bradycardia usually indicates drug toxicity or a failure of the body's compensatory systems.
Scenario
An elderly client with known heart failure, who has previously been stable with lisinopril and metoprolol XL, comes into the clinic with an increase in weight and peripheral edema extending halfway up the left calf.
The client in the scenario with chronic heart failure asks the nurse what else they can do besides drug therapy to control the disease process. Which statement by the nurse would be appropriate? Select all that apply
Explanation
Chronic heart failure involves progressive myocardial dysfunction and neurohormonal activation leading to pulmonary and systemic venous congestion. Management requires strict sodium restriction to minimize fluid retention and exercise tolerance optimization. Secondary complications include pulmonary hypertension, pleural effusions, and cardiorenal syndrome.
Rationale for correct answers
A. Physical activity helps maintain functional capacity and prevents the rapid deconditioning common in elderly cardiac patients. The nurse correctly advises the client to remain active and participate in regular exercise as tolerated by their hemodynamic status. Consistent movement improves peripheral oxygen extraction and reduces the workload on the failing myocardium during daily activities. Exercise should be paced to avoid acute symptomatic exacerbation.
B. Medical nutrition therapy focusing on sodium restriction is essential to prevent the expansion of extracellular fluid volume and worsening edema. A diet low in fat and calories helps manage metabolic demand and reduces the risk of atherosclerotic progression in chronic heart failure. Restricting sodium intake to < 2000 mg per day is a standard clinical guideline for these patients. These dietary modifications directly support the efficacy of prescribed diuretic and antihypertensive medications.
C. Myocardial efficiency is severely compromised in heart failure, necessitating careful balance between activity and rest to prevent cardiac exhaustion. The nurse emphasizes the importance of energy conservation to ensure that the patient does not reach a state of overwhelming fatigue. Avoiding excessive physical stress helps maintain a stable cardiac output and prevents acute decompensation episodes. This lifestyle adjustment is a cornerstone of self-management for chronic cardiovascular disease.
Rationale for incorrect answers
D. Salt substitutes often contain high concentrations of potassium chloride, which can lead to life-threatening levels of serum potassium. Since the client is already taking lisinopril, an ACE inhibitor that causes potassium retention, adding salt substitutes increases the risk of hyperkalemia. Excessive potassium can cause cardiac standstill or lethal ventricular dysrhythmias in elderly patients with reduced renal clearance. The nurse must warn against these substitutes to maintain electrolyte homeostasis.
E. High intake of free water can lead to dilutional hyponatremia and worsening peripheral or pulmonary edema in fluid-overloaded heart failure patients. Alcohol acts as a potent myocardial depressant and can further weaken the contractile force of the heart muscle. Restricting fluids to 1.5 to 2 liters per day is typically required for symptomatic clients like the one described. Consuming multiple alcoholic drinks per day is strictly contraindicated in the management of chronic cardiomyopathy.
Test-taking strategy
- Identify the client condition: The scenario describes an elderly patient with chronic heart failure experiencing current fluid volume excess (weight gain, calf edema).
- Apply safety priorities: Look for interventions that promote hemodynamic stability and prevent electrolyte imbalances.
- Evaluate nutritional safety:
- Recognize that ACE inhibitors (lisinopril) carry a risk of hyperkalemia, making salt substitutes (Choice 4) dangerous.
- Identify that fluid and alcohol restriction (Choice 5) are necessary to prevent further myocardial depression and volume overload.
- Use standard failure management:
- Choice 1 promotes circulation and conditioning.
- Choice 2 follows the American Heart Association guidelines for sodium and lipid control.
- Choice 3 aligns with the nursing goal of reducing cardiac workload.
- Analyze select all that apply format: Treat each statement as an independent true or false clinical fact relevant to the scenario's specific drug regimen and symptoms.
Take home points
- Non-pharmacological management of heart failure centers on sodium restriction, weight monitoring, and balancing activity with rest to prevent cardiac strain.
- Patients taking ACE inhibitors or potassium-sparing diuretics must strictly avoid salt substitutes because the high potassium content can cause fatal hyperkalemia.
- Fluid volume management requires daily weight checks, and an increase of 2 to 3 pounds in 24 hours must be reported to the provider immediately.
- Alcohol should be avoided as it is a cardiotoxin that decreases myocardial contractility and can exacerbate the progression of heart failure.
Scenario
An elderly client with known heart failure, who has previously been stable with lisinopril and metoprolol XL, comes into the clinic with an increase in weight and peripheral edema extending halfway up the left calf.
The client asks the nurse to explain the idea of what is meant by the body’s compensatory mechanisms that occur in response to decreases in heart function. The nurse responds with which correct statement?
Explanation
Compensatory mechanisms in heart failure involve a complex neurohormonal response mediated by the sympathetic nervous system and the renin-angiotensin-aldosterone system. These pathways facilitate hemodynamic stabilization through systemic vasoconstriction and fluid retention. Chronic activation eventually leads to maladaptive ventricular remodeling, further decreasing myocardial efficiency and stroke volume.
Rationale for correct answer
C. Decreased cardiac output triggers the baroreceptor reflex, which activates the sympathetic nervous system to elevate the heart rate and systemic vascular resistance. This immediate response maintains blood pressure and vital organ perfusion in the short term. However, the resulting increase in myocardial oxygen demand and afterload eventually exhausts the heart's functional reserve. Persistent activation leads to clinical decompensation, manifesting as pulmonary congestion and severe exertional fatigue. This physiological trade-off explains the transition from compensated to overt clinical heart failure.
Rationale for incorrect answers
A. Heart failure is characterized by a significant decrease in energy availability for cellular processes rather than an increase. The body’s metabolism is strained by the high oxygen demands of a failing heart and low systemic oxygen delivery. Patients typically experience profound lethargy and exhaustion as the cardiac output fails to meet metabolic needs. There is no biological mechanism in the failure syndrome that generates extra energy for the patient.
B. Compensation in heart failure involves the activation of the renin-angiotensin-aldosterone system, which causes fluid retention rather than increased urination. The kidneys respond to low perfusion by conserving sodium and water, which expands the intravascular volume and increases preload. Increased urination is typically a result of diuretic therapy rather than a natural compensatory response of the body. Reduced urine output or oliguria is more common as renal perfusion pressure declines during decompensation.
D. In heart failure, the body prioritizes blood flow to the brain and heart, leading to a decrease in peripheral perfusion to the skin and limbs. Systemic vasoconstriction shunts blood away from the periphery, causing symptoms like cool extremities and delayed capillary refill. An increase in peripheral blood flow would contradict the body's survival mechanism of maintaining central perfusion pressure. Chronic low flow to the periphery contributes to the muscle wasting seen in advanced stages.
Test-taking strategy
- Identify the core concept: The question asks for the definition of compensatory mechanisms specifically in the context of heart failure.
- Recall autonomic responses: Remember that the initial response to low cardiac output is "fight or flight," which involves the sympathetic nervous system.
- Analyze the physiological outcomes:
- Sympathetic activation always increases heart rate and blood pressure.
- Chronic activation leads to the symptoms mentioned in the scenario, such as edema and weight gain.
- Rule out non-compensatory findings:
- Compensation is an internal struggle to fix a problem, not an "increase in energy" (Choice 1).
- Heart failure causes water retention (edema), which is the opposite of "increase in urination" (Choice 2).
- Poor perfusion is a result of the failure, not a "compensatory increase" (Choice 4).
- Select the balanced perspective: Choice 3 is the most scientifically accurate because it describes the initial attempt to help and the eventual failure of that attempt.
- Use medical logic: Compensation in the cardiovascular system almost always involves adjusting the rate (HR) and the pressure (BP).
Take home points
- The sympathetic nervous system is the first compensatory mechanism to respond to decreased heart function by increasing heart rate and myocardial contractility.
- Activation of the renin-angiotensin-aldosterone system leads to sodium and water retention, which increases preload but eventually causes systemic and pulmonary edema.
- Chronic neurohormonal compensation is ultimately harmful, as it causes ventricular hypertrophy and remodeling that further damages the heart muscle.
- Effective heart failure treatment involves medications like ACE inhibitors and beta blockers that specifically inhibit these maladaptive compensatory pathways.
Practice Questions 2
A client with heart failure has an order for lisinopril (Prinivil, Zestril). Which of the following conditions in the client’s history would lead the nurse to confirm the order with the provider?
Explanation
Lisinopril is an angiotensin-converting enzyme inhibitor that prevents conversion of angiotensin 1 to angiotensin 2, facilitating potent vasodilation. It manages heart failure by reducing afterload, though it may cause a life-threatening angioedema reaction. Serum creatinine and potassium must be monitored regularly during therapy.
Rationale for correct answer
C. Lisinopril is strictly contraindicated in patients with a history of angioedema related to previous ACE inhibitor therapy. This adverse reaction involves rapid submucosal swelling that can cause fatal airway obstruction. Because enalapril and lisinopril belong to the same pharmacological class, cross-sensitivity is a significant risk. This hypersensitivity reaction is mediated by increased levels of bradykinin in the tissues. Safety protocols dictate that this history is an absolute contraindication for all medications in this class.
Rationale for incorrect answers
A. A history of hypertension is a primary indication for lisinopril rather than a reason to question the order. While previous diuretic therapy can increase the risk of first-dose hypotension, it does not prohibit lisinopril use. The nurse should simply monitor blood pressure closely during the initial administration. This combination is a standard treatment for chronic cardiac failure.
B. Seasonal allergies and antihistamine use do not interact negatively with the mechanism of angiotensin-converting enzyme inhibitors. These conditions involve a different immune pathway than the bradykinin-mediated swelling seen with ACE inhibitors. There is no evidence suggesting that lisinopril would exacerbate or be affected by these allergies. The medication remains safe and effective for this specific patient population.
D. Current abstinence from alcoholism does not present a contraindication for lisinopril therapy in the management of heart failure. While active alcohol consumption can cause vasodilation and worsen hypotension, a history of abuse is not a clinical barrier. The nurse should continue to support abstinence while administering the prescribed cardiac medications. Lisinopril does not have a high risk of hepatic toxicity.
Test-taking strategy
- Identify the medication class: Recognize lisinopril and enalapril as ACE inhibitors, which typically share similar side effect profiles and contraindications.
- Prioritize life-threatening risks: Look for the answer choice that represents the greatest threat to patient safety, which is airway compromise.
- Analyze contraindications:
- Distinguish between relative risks (like previous diuretic use) and absolute contraindications (like angioedema).
- Recognize that "history of angioedema" is a red-flag phrase in pharmacology questions involving the "pril" drug class.
- Evaluate cross-sensitivity: Understand that if a patient reacts poorly to one drug in a class (enalapril), they are highly likely to react to others (lisinopril).
- Rule out unrelated conditions: Eliminate choices like seasonal allergies or alcoholism, as they do not significantly impact the safety profile of ACE inhibitors.
- Focus on nursing action: Questioning the order is the correct step when an absolute contraindication is present in the medical record.
Take home points
- Angioedema is a rare but life-threatening side effect of ACE inhibitors that requires immediate discontinuation and permanent avoidance of the drug class.
- Lisinopril works by inhibiting the conversion of angiotensin 1 to angiotensin 2, thereby reducing systemic vascular resistance and cardiac workload.
- Hyperkalemia and a persistent dry cough are common side effects that result from the accumulation of potassium and bradykinin, respectively.
- Nurses must assess renal function and baseline blood pressure before administration, as ACE inhibitors can cause significant hypotension and acute kidney injury.
A client with heart failure is on Lisinopril (Prinivil) as part of the treatment regimen. The nurse monitors the client for the development of which of the following adverse effects of this drug? Select all that apply
Explanation
Lisinopril is an angiotensin-converting enzyme inhibitor that suppresses the renin-angiotensin-aldosterone system to facilitate vasodilation. It treats heart failure by reducing afterload, though it frequently causes hyperkalemia or a dry, nonproductive cough due to bradykinin accumulation.
Rationale for correct answers
A. ACE inhibitors suppress aldosterone secretion, which leads to the retention of potassium ions by the kidneys. This physiological shift commonly results in hyperkalemia, defined as serum potassium levels > 5.0 mEq/L. The nurse must monitor electrolyte panels to prevent subsequent cardiac dysrhythmias. Maintaining normal renal clearance is vital for avoiding dangerous potassium accumulation during therapy. Continuous assessment ensures patient safety regarding cardiac conduction.
C. A persistent, dry, and nonproductive cough is a hallmark side effect caused by the accumulation of bradykinin in the respiratory tract. This inflammatory mediator increases when the converting enzyme is blocked, leading to significant pulmonary irritation. It often necessitates a change to an angiotensin receptor blocker if the symptom becomes intolerable for the client. The cough typically resolves within weeks of discontinuing the offending pharmacological agent. This side effect is unique to the ACE inhibitor class.
D. The potent vasodilatory effect of lisinopril causes a rapid reduction in systemic vascular resistance, which frequently manifests as dizziness. This symptom is especially prevalent after the initial dose or during positional changes, indicating a state of orthostatic hypotension. Clients are advised to move slowly to prevent syncope and potential injury from falling. Monitoring blood pressure ensures the dosage is appropriate for the client's current hemodynamic status. Education on fall prevention is a primary nursing priority.
Rationale for incorrect answers
B. Lisinopril does not significantly influence the renal handling or serum concentration of calcium, making hypocalcemia an unlikely adverse effect. Calcium homeostasis is primarily regulated by parathyroid hormone and vitamin D rather than the renin-angiotensin-aldosterone system. There is no clinical evidence suggesting that ACE inhibitors disrupt the balance of this specific electrolyte. The nurse should focus on potassium and sodium levels instead.
E. While some medications cause gastric irritation, heartburn or gastroesophageal reflux is not a recognized or common adverse effect of lisinopril therapy. Gastrointestinal side effects for this drug class are generally limited to taste disturbances or rare cases of intestinal angioedema. Heartburn is more frequently associated with nonsteroidal anti-inflammatory drugs or certain bisphosphonates. It is not a priority assessment for patients starting a new ACE inhibitor regimen.
Test-taking strategy
- Identify the pharmacological class: Recognize lisinopril as an ACE inhibitor by the "pril" suffix, which is associated with specific physiological effects.
- Link mechanism to electrolytes: Understand that blocking aldosterone leads to potassium retention, making hyperkalemia a high-priority assessment (Choice 1).
- Recall class-specific respiratory effects: Remember that ACE inhibitors uniquely prevent the breakdown of bradykinin, leading to the classic dry cough (Choice 3).
- Prioritize hemodynamic safety: Vasodilators lower blood pressure, which logically leads to dizziness or lightheadedness upon standing (Choice 4).
- Rule out unrelated labs: Calcium (Choice 2) is not part of the RAAS pathway, and gastrointestinal symptoms like heartburn (Choice 5) are not part of the drug's core side effect profile.
- Use the select all that apply mindset: Evaluate each choice independently as a true or false statement regarding the known adverse reactions of lisinopril.
Take home points
- Lisinopril-induced hyperkalemia requires regular monitoring of serum potassium levels to prevent life-threatening cardiac conduction disturbances.
- A persistent, nonproductive cough is a common reason for drug discontinuation and results from the accumulation of inflammatory bradykinins in the lungs.
- First-dose hypotension and dizziness are common, necessitating education on slow position changes to minimize the risk of orthostatic syncope and falls.
- ACE inhibitors are contraindicated in pregnancy due to teratogenic risks and in patients with a history of angioedema or bilateral renal artery stenosis.
A client is taking a beta blocker as part of the treatment plan for heart failure. The nurse knows that the purpose of the beta blocker for this patient is to
Explanation
Beta blockers are sympatholytic agents that competitively antagonize adrenergic receptors to mitigate the deleterious effects of chronic neurohormonal activation. They manage congestive heart failure by reducing myocardial oxygen demand and preventing pathological remodeling of the ventricles. Standard parameters for administration include maintaining a heart rate ≥ 60 beats/min and systolic blood pressure ≥ 90 mmHg.
Rationale for correct answer
B. Chronic heart failure triggers a sustained sympathetic nervous system response, resulting in excessive levels of circulating catecholamines like norepinephrine. These substances cause progressive cardiac damage and lethal arrhythmias through constant adrenergic stimulation. Beta blockers function by occupying the receptor sites, effectively blocking these stimulatory signals from reaching the myocardium. This blockade allows the heart to rest, improves diastolic filling time, and significantly reduces the long-term mortality associated with ventricular failure.
Rationale for incorrect answers
A. Beta blockers do not possess diuretic properties and do not directly act on the renal tubules to increase urine output. Increased diuresis is the primary therapeutic goal of agents like loop diuretics or aldosterone antagonists. While improved cardiac output may eventually enhance renal perfusion, beta blockers themselves do not facilitate fluid excretion. They are primarily used for their chronotropic and inotropic modulating effects.
C. Beta blockers are negative inotropes, which means they actually decrease contractility rather than increase it during the initial phase of treatment. Increasing the force of contraction is the mechanism of action for positive inotropic agents such as digoxin or dobutamine. Because beta blockers reduce the vigor of myocardial shortening, they must be started at very low doses in heart failure. This prevents an acute exacerbation of heart failure symptoms during the initiation of therapy.
D. Most standard beta blockers used in heart failure, such as metoprolol succinate, do not cause significant peripheral vasodilation as their primary action. Direct relaxation of vascular smooth muscle is the function of vasodilators like hydralazine or nitrates. While certain non-selective agents like carvedilol have alpha-blocking properties that assist with vasodilation, the fundamental purpose of the class is heart rate and rhythm control. The goal is central cardiac protection rather than systemic vascular resistance reduction.
Test-taking strategy
- Identify the pathophysiology of heart failure: Recognize that the body compensates for a failing heart by increasing sympathetic tone, which releases norepinephrine.
- Understand drug class mechanisms: Beta blockers "block" the beta-adrenergic receptors from being stimulated by endogenous chemicals.
- Distinguish between short-term and long-term goals:
- Short-term, beta blockers may decrease heart performance (negative inotropy).
- Long-term, they protect the heart from the toxic effects of catecholamines (Choice 2).
- Rule out other drug classes:
- Diuretics increase urine (Choice 1).
- Inotropes increase contractility (Choice 3).
- Vasodilators/ACE inhibitors affect the periphery (Choice 4).
- Focus on the neurohormonal hypothesis: Modern heart failure treatment focuses on blocking the hormonal systems that damage the heart over time, making "prevention of stimulation" the most scientifically accurate purpose.
- Analyze the nomenclature: The term "blocker" implies an inhibitory action, which matches the concept of "preventing stimulation."
Take home points
- Beta blockers are used in chronic heart failure to block the cardiotoxic effects of catecholamines and prevent maladaptive ventricular remodeling.
- These agents exert negative chronotropic and inotropic effects, requiring the nurse to monitor for bradycardia and worsening fluid retention during dose titration.
- Long-term benefits include increased ejection fraction, reduced hospitalization rates, and a significant decrease in the risk of sudden cardiac death.
- Beta blockers must be initiated only when the patient is stable and euvolemic, as they can temporarily worsen heart failure if started during an acute decompensation.
A client with heart failure will be starting the beta blocker metoprolol (Lopressor). The nurse will monitor for which expected cardiovascular effects? Select all that apply
Explanation
Metoprolol is a selective beta-1 antagonist that competitively inhibits catecholamine binding at myocardial receptors to mitigate chronic neurohormonal activation. It manages heart failure by reducing metabolic demand and preventing ventricular remodeling through negative chronotropic and inotropic effects.
Rationale for correct answers
C. Metoprolol suppresses the conduction velocity of electrical impulses traveling through the atrioventricular junction, resulting in delayed conduction. This dromotropic effect helps control ventricular rate, especially in patients with coexisting supraventricular tachyarrhythmias or atrial fibrillation. The nurse monitors the PR interval on the electrocardiogram to ensure the delay does not progress to a high-grade heart block. This effectively reduces the workload of the failing heart by regulating impulse transmission.
D. By antagonizing beta-1 receptors in the sinoatrial node, metoprolol produces a significant reduced rate of discharge. This negative chronotropic effect increases diastolic filling time, which enhances coronary artery perfusion and myocardial oxygenation in heart failure patients. The nurse must assess the apical pulse for 1 full minute, typically withholding the medication if the rate is < 60 beats/min. Lowering the heart rate is a primary goal to prevent tachycardia-induced cardiomyopathy.
E. Beta-adrenergic blockade leads to decreased automaticity within the pacemaker cells of the heart, reducing the likelihood of ectopic impulse formation. This inhibitory action on the spontaneous depolarization of cardiac fibers helps prevent life-threatening ventricular or atrial dysrhythmias common in congestive heart failure. The nurse observes the telemetry monitor for a reduction in premature contractions as a sign of drug efficacy.
Rationale for incorrect answers
A. Metoprolol is a sympatholytic agent that blocks adrenergic stimulation, therefore it never results in an increased rate of cardiac contraction. An elevation in heart rate would be the result of a beta-agonist like epinephrine or an anticholinergic like atropine. Tachycardia would oppose the therapeutic intent of reducing myocardial oxygen consumption in a failing heart.
B. As a negative inotrope, metoprolol causes a decrease in the force of contraction rather than increased contractility in the myocardium. Positive inotropic effects are achieved through cardiac glycosides like digoxin or phosphodiesterase inhibitors which increase intracellular calcium availability. In heart failure, the initial decrease in contractility requires cautious up-titration of the dose to avoid acute decompensation.
Test-taking strategy
- Identify the medication class: Recognize metoprolol as a beta-1 selective blocker, which primarily affects the heart by inhibiting sympathetic stimulation.
- Apply physiological nomenclature: Recall the "negative" properties of beta blockers, which include negative chronotropy (rate), negative dromotropy (conduction), and negative inotropy (contractility).
- Evaluate the cardiovascular effects:
- If the drug is a "blocker" of the sympathetic system, it must "decrease" or "delay" cardiac functions rather than increase them.
- This principle allows for the immediate elimination of Choice 1 and Choice 2, which describe stimulatory effects.
- Match clinical findings to choices:
- Choice 3 (delayed conduction) and Choice 4 (reduced rate) are direct results of node inhibition.
- Choice 5 (decreased automaticity) is a standard electrophysiological result of adrenergic blockade.
- Use the select all that apply approach: Treat each option as a true/false statement based on the core pharmacology of beta-1 antagonists.
- Consider the patient population: In heart failure, these negative effects are utilized to prevent the heart from "burning out" due to chronic overstimulation.
Take home points
- Metoprolol provides cardiac protection in heart failure by blocking the toxic effects of chronic catecholamine exposure on the myocardium.
- Expected cardiovascular findings include a reduced heart rate and delayed AV conduction, necessitating baseline assessment of pulse and rhythm.
- The negative inotropic effect can temporarily worsen heart failure symptoms, so patients must be monitored for increased dyspnea or peripheral edema during initiation.
- Beta-1 selectivity reduces the risk of bronchospasm compared to non-selective agents, but caution is still required in patients with reactive airway diseases.
A nurse is reviewing lab results for a client starting sacubitril/valsartan. Which lab finding requires immediate attention?
Explanation
Sacubitril/valsartan is an angiotensin receptor-neprilysin inhibitor that increases natriuretic peptides while blocking the renin-angiotensin-aldosterone system. This combination therapy manages heart failure by inducing vasodilation and reducing sympathetic outflow. Adverse effects include hyperkalemia, hypotension, and angioedema. It is contraindicated with concurrent ACE inhibitor use or a history of hereditary angioedema.
Rationale for correct answer
B. Valsartan, the ARB component, suppresses aldosterone secretion, which leads to significant renal retention of potassium ions. A serum level of 5.8 mEq/L represents moderate hyperkalemia, exceeding the physiological limit of 5.0 mEq/L. This biochemical state poses an immediate risk for lethal cardiac arrhythmias and sinus bradycardia. The nurse must prioritize this finding to prevent cardiovascular collapse.
Rationale for incorrect answers
A. The reported sodium level of 138 mEq/L falls within the normal reference range of 135 to 145 mEq/L. While diuretics and ARBs can cause hyponatremia, this value indicates a stable homeostatic balance of extracellular fluid. There is no evidence of a sodium deficit or excess in this client. This finding does not warrant immediate nursing or medical intervention.
C. A creatinine level of 1.0 mg/dL is within the standard expected range for an adult, indicating adequate glomerular filtration. Although ARBs can occasionally cause a transient rise in creatinine, this specific value suggests normal nephron function. It does not indicate the presence of acute kidney injury or chronic renal failure. The nurse would simply continue to monitor this value periodically.
D. The BUN level of 16 mg/dL is well within the normal parameters of 7 to 20 mg/dL. This indicates that the client has appropriate nitrogenous waste clearance and is likely in a euvolemic state. It shows no signs of the prerenal azotemia sometimes associated with aggressive diuresis or heart failure exacerbation. This laboratory result is considered a benign finding during heart failure therapy.
Test-taking strategy
- Identify the drug class: Sacubitril/valsartan contains an ARB. Recall that ARBs and ACE inhibitors carry a primary risk of elevated potassium.
- Analyze lab values against normal ranges:
- Compare 5.8 mEq/L potassium to the normal 3.5 to 5.0 range. This is the only abnormal value provided.
- Compare 138 mEq/L sodium to 135 to 145. This is normal.
- Compare 1.0 mg/dL creatinine and 16 mg/dL BUN to standard references. Both are normal.
- Prioritize based on safety: Hyperkalemia is a high-acuity finding because of its direct impact on myocardial electrical stability.
- Rule out distractors: Use the process of elimination to discard any values that are within standard limits.
- Focus on the mechanism: Because valsartan blocks aldosterone, the kidney cannot "trade" sodium for potassium, leading to potassium accumulation.
Take home points
- Sacubitril/valsartan is a first-line treatment for heart failure with reduced ejection fraction but requires strict monitoring of serum potassium.
- Hyperkalemia is a critical adverse effect that can lead to peaked T waves, widened QRS complexes, and eventual cardiac arrest.
- The medication should be held, and the provider notified, if serum potassium levels exceed 5.0 to 5.5 mEq/L to ensure patient safety.
- Renal function tests and electrolytes should be checked within one to two weeks after initiating therapy or increasing the dosage.
A nurse is teaching a client who has heart failure and is prescribed sacubitril/valsartan. Which of the following instructions should the nurse include?
Explanation
Sacubitril/valsartan is an angiotensin receptor-neprilysin inhibitor that increases natriuretic peptides while blocking the renin-angiotensin-aldosterone system. This combination therapy manages heart failure by inducing vasodilation and reducing sympathetic outflow. Adverse effects include hyperkalemia, hypotension, and angioedema. It is contraindicated with concurrent ACE inhibitor use or a history of hereditary angioedema.
Rationale for correct answer
B. Sacubitril/valsartan induces significant systemic vasodilation and natriuresis to reduce cardiac workload. These pharmacological actions frequently result in symptomatic hypotension, which can cause dizziness or syncope. Regular blood pressure monitoring is vital to ensure hemodynamic stability and to adjust dosages appropriately. This assessment helps the nurse identify if the patient is responding safely to the drug's antihypertensive effect.
Rationale for incorrect answers
A. Combining this medication with an ACE inhibitor like lisinopril significantly increases the risk of life-threatening angioedema. A strict washout period of 36 hours is mandatory when transitioning between these two drug classes. Concurrent administration is strictly contraindicated to prevent severe airway obstruction from bradykinin accumulation.
C. Valsartan inhibits aldosterone secretion, which directly leads to the renal retention of potassium. Using potassium supplements or salt substitutes containing potassium chloride can precipitate severe, lethal hyperkalemia. Maintaining a serum potassium level within 3.5 to 5.0 mEq/L is essential for myocardial stability.
D. Drugs that act directly on the renin-angiotensin-aldosterone system carry a black box warning for fetal toxicity. Exposure during the second and third trimesters can cause fetal injury or death due to impaired renal development. This medication is not safe for use during pregnancy and must be discontinued immediately if conception occurs. Female clients of childbearing age require education on effective contraception methods.
Test-taking strategy
- Identify the medication class: Recognize sacubitril/valsartan as an ARNI. Its primary functions are to lower blood pressure and manage fluid volume in heart failure.
- Evaluate safety protocols:
- Rule out option 1: Recall the mandatory 36-hour washout period between ACE inhibitors and ARNIs to prevent angioedema.
- Rule out option 3: Recall that ARBs (valsartan) cause potassium retention, making supplements dangerous.
- Rule out option 4: Identify that RAAS-targeting drugs are notorious teratogens and are contraindicated in pregnancy.
- Select the monitoring priority: Blood pressure (option 2) is a standard, essential assessment for any patient on a potent vasodilator to prevent orthostatic injury.
- Focus on the nursing process: Teaching a patient to monitor the drug's primary effect (lowered pressure) is a foundational nursing intervention for medication safety.
Take home points
- Daily blood pressure monitoring is essential for patients on sacubitril/valsartan to detect and manage medication-induced hypotension.
- A 36-hour washout period is required when switching from an ACE inhibitor to prevent the high risk of life-threatening angioedema.
- Potassium-rich foods and supplements must be limited to avoid hyperkalemia, which can cause dangerous changes in cardiac rhythm.
Sacubitril/valsartan is contraindicated in pregnancy due to the high risk of serious birth defects and fetal death.
Practice Questions 3
A nurse is caring for an older adult client who has a new prescription for digoxin and takes multiple other medications. Concurrent use of which of the following medications places the client at risk for digoxin toxicity?
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to increase intracellular calcium. This positive inotrope is indicated for heart failure and atrial fibrillation but has a narrow therapeutic index (0.5 to 2.0 ng/mL). Toxicity often manifests as xanthopsia or life-threatening arrhythmias secondary to electrolyte disturbances.
Rationale for correct answer
B. Verapamil is a calcium channel blocker that significantly inhibits P-glycoprotein-mediated efflux of digoxin. This interaction leads to a reduced renal clearance and increased serum concentrations of digoxin. Clinical monitoring is essential to prevent digoxin toxicity when these agents are used concurrently. It can raise serum levels by 70% to 100%.
Rationale for incorrect answers
A. Phenytoin is an anticonvulsant that may actually decrease serum digoxin levels by inducing hepatic metabolism. It is sometimes used to treat specific digoxin-induced arrhythmias rather than causing toxicity. This medication does not increase the risk of drug accumulation for digitalis. It typically functions as an enzyme inducer in the liver.
C. Warfarin is an oral anticoagulant that primarily inhibits vitamin K-dependent clotting factors. While both drugs require frequent monitoring, warfarin does not alter the pharmacokinetics or clearance of digoxin. There is no direct evidence that it precipitates toxic levels of cardiac glycosides. Monitoring focuses on the International Normalized Ratio instead.
D. Aluminum hydroxide is an antacid that can interfere with the gastrointestinal absorption of digoxin. This interaction generally leads to decreased bioavailability rather than toxicity. Patients are advised to space these medications to ensure therapeutic efficacy is maintained. Lowered serum levels are the typical clinical result of this co-administration.
Test-taking strategy
- Identify the central pharmacological problem: The question asks for a drug-drug interaction that increases serum levels to a toxic range.
- Apply the principle of P-glycoprotein inhibition: Recognize that certain classes, specifically non-dihydropyridine calcium channel blockers like Verapamil, are notorious for increasing digitalis levels.
- Rule out absorption inhibitors: Antacids like Aluminum hydroxide decrease absorption, which leads to subtherapeutic levels rather than toxicity.
- Differentiate metabolic effects: Phenytoin is an inducer, which lowers levels, while Warfarin has a separate metabolic pathway involving Vitamin K, not the sodium-potassium pump.
- Focus on the narrow therapeutic window: In older adults, renal function is often diminished, making them more susceptible to any drug that decreases renal clearance or alters efflux proteins.
Take home points
- Digoxin toxicity is frequently precipitated by drugs that inhibit P-glycoprotein, such as verapamil, amiodarone, and quinidine.
- Hypokalemia sensitizes the myocardium to digoxin, so diuretics should be monitored closely alongside digitalis therapy.
- Clinical manifestations of toxicity include visual disturbances, nausea, vomiting, and various degrees of heart block.
- Therapeutic drug monitoring is mandatory when introducing or removing medications that alter the renal excretion of digoxin.
A nurse is providing teaching to a client who has a new prescription for digoxin (Lanoxin). Which of the following may indicate digoxin toxicity and should be reported to the provider? Select all that apply
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump, increasing intracellular calcium to treat atrial fibrillation. It has a narrow therapeutic index (0.5-2 ng/mL), and toxicity is exacerbated by hypokalemia, causing dysrhythmias and neurological disturbances.
Rationale for correct answers
A. Central nervous system effects are early markers of toxicity. The client may experience significant fatigue or generalized weakness. This occurs because the drug affects neural transmission. These neurological symptoms often precede severe cardiac complications. Prompt identification of lethargy is essential for patient safety.
C. Gastrointestinal distress is a classic sign of digitalis overdose. The client typically develops anorexia, nausea, or vomiting. These symptoms result from direct stimulation of the chemoreceptor trigger zone. Early recognition prevents further drug accumulation. This sign often presents before cardiac rhythm changes occur.
E. Visual disturbances are pathognomonic for high serum digoxin levels. Patients may report diplopia, blurred vision, or yellow-green halos. These ophthalmologic changes indicate significant toxicity. Reporting these immediately is vital to prevent life-threatening ventricular arrhythmias. Vision changes result from inhibition of retinal sodium-potassium pumps.
Rationale for incorrect answers
B. Digoxin toxicity does not typically cause constipation in patients. Instead, gastrointestinal toxicity more frequently manifests as diarrhea or abdominal pain. Constipation is a common side effect of calcium channel blockers. It is unrelated to digoxin levels.
D. The development of a rash is not a standard sign of digoxin toxicity. Dermatological reactions might indicate a rare drug allergy but not toxicity. Toxicity focuses on cardiac, gastrointestinal, and neurological systems. Rashes are more common with antibiotics.
Test-taking strategy
- Identify the therapeutic range: Recognize that digoxin has a narrow margin of safety, requiring close monitoring of serum levels (0.5-2 ng/mL).
- Categorize symptoms: Group toxicity signs into three main categories: gastrointestinal (anorexia, nausea), neurological/visual (fatigue, halos, diplopia), and cardiac (bradycardia, heart blocks).
- Use the process of elimination:
- Eliminate Choice 2 because constipation is generally associated with decreased motility or other drug classes like opioids.
- Eliminate Choice 4 because integumentary changes are rarely linked to serum digitalis concentrations.
- Prioritize early signs: Recall that gastrointestinal symptoms like anorexia (Choice 3) are often the very first indicators of toxicity.
- Link physiology to symptoms: Understand that ATPase inhibition in non-cardiac tissues leads to the neurological (Choice 1) and visual (Choice 5) manifestations.
- Check for electrolyte interactions: Always remember that hypokalemia increases the risk of toxicity even if the digoxin level is within the normal range.
Take home points
- Digoxin toxicity presents with gastrointestinal symptoms such as anorexia, nausea, and vomiting as the earliest indicators.
- Neurological and visual manifestations include profound fatigue, confusion, diplopia, and the classic perception of yellow-green halos around lights.
- Cardiac manifestations are serious and include bradycardia, varying degrees of heart block, and ventricular dysrhythmias.
- Monitor serum potassium levels closely because low potassium increases digoxin binding to the ATPase pump, significantly raising the risk of toxicity.
A nurse is monitoring the digoxin level for a client who has been taking a daily dose of digoxin for 1 month. The digoxin level is 0.25 ng/mL. The nurse should notify the provider and anticipate which of the following?
Explanation
Digoxin is a cardiac glycoside that reversibly inhibits the sodium-potassium ATPase pump to increase intracellular calcium. It provides positive inotropic effects while decreasing heart rate through vagal stimulation for congestive heart failure management. Therapeutic serum levels typically range from 0.5 to 2 ng/mL. Toxicity occurs above 2.4 ng/mL, requiring antibody fragments for reversal.
Rationale for correct answer
A. The client's level of 0.25 ng/mL falls significantly below the therapeutic range of 0.5 to 2 ng/mL. This subtherapeutic concentration indicates the current dose is insufficient to achieve the desired clinical effect on myocardial contractility. The nurse must anticipate an increase in the dosage to reach therapeutic efficacy. Providing a higher dose ensures the inhibition of the ATPase pump is adequate for hemodynamic support.
Rationale for incorrect answers
B. Decreasing the dose is inappropriate because the current level is already below the minimum therapeutic threshold for efficacy. A reduction would further lower the serum concentration, rendering the medication completely ineffective. This action would lead to decompensated heart failure.
C. Maintaining the current dose would result in a continued subtherapeutic state for the patient. Since the level is only 0.25 ng/mL, the medication is not providing the necessary inotropic support. Providers must titrate the dose until the steady state level reaches the 0.5 to 2 ng/mL window.
D. Discontinuation is only indicated in cases of severe toxicity or hypersensitivity reactions. The current lab value shows a lack of drug rather than an excess or an adverse reaction. Stopping the medication would deprive the patient of hemodynamic benefits once the dose is properly adjusted.
Test-taking strategy
- Memorize therapeutic ranges: For digoxin, the standard range is 0.5 to 2 ng/mL; levels below 0.5 are subtherapeutic, and levels above 2 are toxic.
- Compare the data: The question provides a value of 0.25 ng/mL, which is numerically less than the minimum 0.5 ng/mL requirement.
- Identify the nursing goal: In this scenario, the goal is to achieve a therapeutic level to manage the patient's heart failure.
- Evaluate the outcomes:
- If the level is low, the dose must go up.
- If the level is high, the dose must go down or be held.
- If the level is normal, no change is required.
- Match the finding to the action: Because the value is low, an increase in dosage is the logical and scientifically sound expectation.
- Consider the steady state: Since the patient has been on the dose for 1 month, this value represents an accurate baseline, necessitating a change in the daily regimen.
Take home points
- The therapeutic reference range for serum digoxin is 0.5 to 2 ng/mL, and levels must be monitored to ensure efficacy and safety.
- A digoxin level below 0.5 ng/mL is considered subtherapeutic and usually requires a dosage increase to achieve desired inotropic effects.
- Clinical manifestations of subtherapeutic levels include worsening peripheral edema, increased dyspnea, and decreased exercise tolerance due to inadequate cardiac output.
- Serum levels should be drawn at least 6 to 8 hours after the last dose to ensure an accurate measurement of the steady state concentration.
The client is prescribed digoxin (Lanoxin) for treatment of heart failure. Which of the following statements by the client indicates the need for further teaching?
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to increase intracellular calcium, promoting positive inotropy. It manages heart failure symptoms by enhancing cardiac output and eliciting a negative chronotropic effect via vagal stimulation. Serum therapeutic levels range from 0.5 to 2 ng/mL. Toxicity presents as cardiac dysrhythmias, xanthopsia, and gastrointestinal distress.
Rationale for correct answer
C. Heart failure is a chronic, progressive syndrome characterized by structural or functional cardiac impairment that is currently incurable. The client expressing that the drug will cure the condition demonstrates a significant knowledge deficit regarding the prognosis and goals of therapy. Digoxin serves purely as a palliative measure to improve hemodynamic stability and reduce hospitalizations.
Rationale for incorrect answers
A. Digoxin exerts a potent vagomimetic effect on the sinoatrial and atrioventricular nodes, which naturally causes the heart rate to slow down. The client correctly identifies this expected negative chronotropic response as a normal outcome of the prescribed medication regimen. This statement requires no further intervention from the nursing staff.
B. Patients often report transient lethargy or fatigue during the initial titration phase as the cardiovascular system adjusts to altered perfusion patterns. This physiological adaptation occurs as the medication works to optimize the stroke volume and cardiac index over several days. The client's statement accurately reflects a common clinical phenomenon during the start of treatment.
D. By increasing the force of myocardial contraction, digoxin effectively increases the cardiac output, which improves oxygen delivery to the peripheral tissues. This hemodynamic improvement typically results in a gradual energy increase and a reduction in the symptoms of exertional dyspnea. The client's expectation of feeling better over time is a scientifically accurate assessment.
Test-taking strategy
- Identify the negative polarity of the question: The phrase "need for further teaching" indicates you must select the statement that is medically incorrect or a misconception.
- Evaluate chronicity vs. cure: Distinguish between chronic disease management and curative treatment. Heart failure is a life-long condition requiring continuous maintenance.
- Analyze drug mechanisms:
- Recall that Digoxin decreases heart rate (eliminates Choice 1).
- Recall that Digoxin increases contractility and output (eliminates Choice 4).
- Assess client expectations: Fatigue (Choice 2) is a documented early side effect as the body achieves a steady-state serum concentration.
- Use the process of elimination: Since statements 1, 2, and 4 are therapeutic goals or known side effects, Choice 3 is the only illogical conclusion regarding disease prognosis.
- Apply scientific facts: No current medication, including cardiac glycosides, is capable of reversing the structural damage (remodeling) associated with heart failure.
Take home points
- Digoxin is a maintenance medication used for symptomatic relief of heart failure and rate control in atrial fibrillation but does not provide a definitive cure for the condition.
- The drug possesses negative chronotropic and dromotropic effects, necessitating the assessment of the apical pulse for 1 full minute prior to administration.
- Improved systemic perfusion resulting from increased myocardial contractility leads to decreased dyspnea and improved functional capacity in the long term.
- Patient education should focus on the chronic nature of heart failure and the importance of monitoring for signs of toxicity, such as visual changes or anorexia.
The nurse reviews laboratory studies of a client receiving digoxin (Lanoxin). Intervention by the nurse is required if the results include which of the following laboratory values?
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to increase intracellular calcium, promoting positive inotropy. It manages heart failure and supraventricular tachyarrhythmias by enhancing vagal tone and slowing atrioventricular conduction. Toxicity risk increases significantly with hypokalemia, defined as potassium levels < 3.5 mEq/L, or hypomagnesemia.
Rationale for correct answer
B. A serum potassium level of 3 mEq/L indicates clinically significant hypokalemia, which dramatically increases the risk of digitalis toxicity. Potassium and digoxin compete for binding sites on the ATPase pump within myocardial cells. When potassium is low, more digoxin molecules bind to the pump, potentially causing lethal dysrhythmias. The nurse must intervene immediately by notifying the provider and anticipating potassium supplementation. This finding is the most critical because it directly alters the pharmacodynamics of the drug.
Rationale for incorrect answers
A. The serum digoxin level of 1.2 ng/dL falls within the standard therapeutic range of 0.5 to 2 ng/dL. This value indicates that the client is receiving an effective dose without reaching toxic concentrations in the bloodstream. No nursing intervention is required as this is an expected laboratory finding for therapeutic management. Monitoring should continue at regular intervals to ensure the level remains stable.
C. A hemoglobin level of 14.4 g/dL is within the normal reference range for an adult client. This value suggests adequate oxygen-carrying capacity and the absence of anemia or hemoconcentration at this time. It does not affect the administration or the physiological action of cardiac glycosides in the heart. The nurse would simply document this result as a baseline finding in the medical record.
D. The serum sodium level of 140 mEq/L is a perfectly normal value, as the typical reference range is 135 to 145 mEq/L. While digoxin affects the sodium-potassium pump, serum sodium fluctuations do not significantly increase the risk of drug toxicity like potassium does. This result indicates proper electrolyte balance and does not necessitate any changes to the current treatment plan. The nurse should proceed with standard care for a stable patient.
Test-taking strategy
- Identify the nursing priority: The question asks for "intervention," which signifies an abnormal finding that poses a safety risk to the client.
- Recall electrolyte interactions: Recognize the inverse relationship between potassium levels and digoxin binding.
- Evaluate normal ranges:
- Digoxin: 0.5 to 2 ng/dL (Choice 1 is normal).
- Potassium: 3.5 to 5.0 mEq/L (Choice 2 is abnormal/low).
- Hemoglobin: 12 to 18 g/dL (Choice 3 is normal).
- Sodium: 135 to 145 mEq/L (Choice 4 is normal).
- Connect the physiology: Hypokalemia (low potassium) is the most dangerous electrolyte imbalance for a patient on digoxin because it sensitizes the myocardium to the drug's effects.
- Use the process of elimination: Since choices 1, 3, and 4 are within normal physiological limits, Choice 2 is the only logical answer requiring immediate clinical action.
- Focus on risk reduction: Prioritize the laboratory value that could lead to life-threatening complications like ventricular fibrillation.
Take home points
- Hypokalemia is a major predisposing factor for digoxin toxicity because low extracellular potassium increases drug binding to the sodium-potassium ATPase pump.
- The therapeutic range for digoxin is 0.5 to 2 ng/dL, and levels above 2.4 ng/dL are generally considered toxic and require monitoring for dysrhythmias.
- Nurses must assess serum potassium, magnesium, and calcium levels frequently, as imbalances in these electrolytes can alter cardiac sensitivity to glycosides.
- Clinical signs of toxicity often include gastrointestinal upset, blurred vision, and bradycardia, even if serum digoxin levels appear to be within range.
Practice Exercise 4
The client who has not responded well to other therapies has been prescribed milrinone (Primacor) for treatment of his heart failure. What essential assessment must the nurse make before starting this drug?
Explanation
Milrinone is a phosphodiesterase-3 inhibitor that increases cyclic adenosine monophosphate levels to improve myocardial contractility and facilitate peripheral vasodilation. It treats decompensated heart failure by providing inodilator effects, though it poses significant risks for life-threatening ventricular dysrhythmias.
Rationale for correct answer
C. Milrinone increases the risk of cardiac arrhythmias, which are significantly exacerbated by electrolyte imbalances. The nurse must assess baseline serum electrolytes, particularly potassium and magnesium, before initiating the intravenous infusion. Correcting hypokalemia is vital because low potassium levels sensitize the myocardium to drug-induced proarrhythmic effects. This assessment directly mitigates the potential for lethal ventricular tachycardia during therapy.
Rationale for incorrect answers
A. Assessing body weight and the presence of edema are standard nursing interventions for heart failure monitoring. However, these baseline measurements do not take precedence over the immediate safety risk of arrhythmogenesis associated with milrinone. Weight reflects fluid volume status rather than the electrophysiological stability required for inotropic therapy. Edema assessment is a secondary indicator of long-term drug efficacy in failure management.
B. Evaluating the sodium intake of a client provides data on nutritional compliance and volume overload risk factors. While sodium restriction is critical in managing congestive heart failure, it is not an essential prerequisite for the administration of milrinone. The drug mechanism focuses on intracellular calcium handling rather than sodium-driven extracellular fluid shifts.
D. Screening for sleep apnea and documenting nocturnal sleep patterns help identify comorbidities that worsen cardiac strain. These findings contribute to the holistic management of chronic failure but are irrelevant to the immediate pharmacodynamics of phosphodiesterase inhibitors. Sleep patterns do not influence the dosing or the acute contraindications of milrinone therapy.
Test-taking strategy
- Identify the medication class: Recognize milrinone as a phosphodiesterase-3 inhibitor used for acute heart failure management.
- Identify the primary safety risk: Recall that inotropic agents like milrinone are associated with a high incidence of cardiac dysrhythmias.
- Prioritize physiological safety:
- Apply the principle that electrolyte stability is fundamental to cardiac electrophysiology.
- Choice 3 addresses the physiological baseline necessary to prevent life-threatening adverse reactions (arrhythmias).
- Evaluate the timing of assessment:
- While weight (Choice 1) and diet (Choice 2) are important for heart failure, they are not "essential" safety checks before starting this specific high-risk drug.
- Choice 4 is a long-term assessment that does not impact the immediate administration of a vasoactive infusion.
- Use clinical reasoning: If a drug can cause the heart to beat irregularly, the nurse must first check the lab values that prevent or cause irregular heartbeats.
Take home points
- Milrinone acts as a positive inotrope and vasodilator by inhibiting phosphodiesterase-3, which increases intracellular cAMP and calcium availability.
- Baseline electrolyte monitoring is mandatory because hypokalemia and hypomagnesemia significantly increase the risk of milrinone-induced ventricular dysrhythmias.
- Continuous hemodynamic monitoring, including blood pressure and electrocardiogram, is required during infusion due to the high risk of hypotension and arrhythmias.
- Milrinone is typically reserved for short-term management of acute decompensated heart failure in patients who do not respond to conventional therapies.
The nurse is assessing a client who is receiving an intravenous milrinone infusion and checks the client’s cardiac rhythm on the heart monitor. What adverse cardiac effect is most likely to occur in the client?
Explanation
Milrinone is a bipyridine phosphodiesterase-3 inhibitor that increases cyclic adenosine monophosphate levels to augment myocardial contractility and facilitate vascular relaxation. It treats decompensated heart failure by increasing stroke volume while decreasing pulmonary capillary wedge pressure through potent systemic vasodilation. Side effects include severe hypotension and life-threatening arrhythmogenesis requiring continuous telemetry.
Rationale for correct answer
D. Milrinone increases intracellular calcium levels in the myocardium, which significantly predisposes the cardiac conduction system to high-frequency ventricular dysrhythmia. Ectopic activity often manifests as premature ventricular contractions or sustained ventricular tachycardia during intravenous administration. This proarrhythmic effect is the most dangerous cardiac complication associated with this pharmacological class. The nurse must monitor the heart rhythm continuously to detect potentially lethal electrical instability.
Rationale for incorrect answers
A. While milrinone might cause a slight increase in heart rate due to its inotropic effects, significant tachycardia is not the primary adverse cardiac finding. The drug possesses some positive chronotropic properties, but these are secondary to its effects on contractility and vasodilation. Ventricular irritability remains a much more common and serious complication than simple sinus rate elevation. Heart rate changes usually remain within manageable clinical limits during therapy.
B. Milrinone therapy does not typically induce bradycardia because its primary mechanism involves increasing rather than decreasing cardiac workload and conduction speed. As a positive inotropic agent, it generally supports or slightly increases the heart rate through electrophysiological stimulation. Slow heart rates are more characteristic of medications like beta-blockers or calcium channel blockers. There is no pharmacological basis for this drug to cause a depressive effect on the sinoatrial node.
C. Although atrial fibrillation is common in the heart failure population, it is not the specific adverse effect most attributed to milrinone. The medication primarily targets ventricular tissues where phosphodiesterase-3 is highly concentrated, leading to ventricular rather than supraventricular irritability. New-onset atrial arrhythmias are less frequent than the development of ventricular ectopy during active infusion. Focus remains on monitoring the ventricles for life-threatening changes in rhythm.
Test-taking strategy
- Identify the drug classification: Recognize milrinone as an inodilator and a phosphodiesterase-3 (PDE3) inhibitor used for short-term failure management.
- Apply pathophysiological knowledge: Understand that agents increasing intracellular calcium and cAMP are inherently proarrhythmic due to increased myocardial excitability.
- Prioritize safety risks:
- Compare the choices to determine which arrhythmia is most life-threatening.
- Choice 4 represents a ventricular event, which always takes priority over atrial or rate-based changes in an acute care setting.
- Eliminate unlikely physiological effects:
- Rule out bradycardia (Choice 2) as it contradicts the stimulatory nature of inotropes.
- Distinguish between tachycardia (Choice 1) and dysrhythmia (Choice 4); while both involve high rates, "dysrhythmia" specifically captures the irregular, dangerous electrical patterns seen with milrinone.
- Use medical patterns: Recall that phosphodiesterase inhibitors are historically linked to increased mortality in long-term use due to sudden cardiac death from ventricular origin.
Take home points
- Milrinone acts as a positive inotrope and vasodilator by inhibiting the breakdown of cAMP, which increases calcium availability for myocardial contraction.
- Ventricular dysrhythmias are the most significant and frequent adverse cardiac effects of milrinone, necessitating constant electrocardiographic monitoring.
- Patients must be monitored for dose-related hypotension, as the drug's vasodilatory effects can significantly reduce systemic vascular resistance and blood pressure.
- Intravenous milrinone is typically indicated only for the short-term treatment of acute decompensated heart failure in patients not responding to standard therapies.
The nurse is administering an intravenous infusion of a phosphodiesterase inhibitor to a client who has heart failure. The nurse will evaluate the client for which therapeutic effects? Select all that apply
Explanation
Phosphodiesterase 3 inhibitors like milrinone facilitate myocardial contractility by preventing the degradation of cyclic adenosine monophosphate in cardiac and vascular smooth muscle. This mechanism enhances calcium influx during systole, producing a potent inotropy and systemic vasodilation often termed inodilatory action. These agents are indicated for short-term management of acute decompensated heart failure when patients exhibit low cardiac output and elevated pulmonary capillary wedge pressure.
Rationale for correct answers
A. Inhibition of the phosphodiesterase enzyme leads to higher concentrations of intracellular cyclic adenosine monophosphate, which increases the availability of calcium. This results in positive inotropic effects characterized by significantly increased myocardial contractility and stroke volume. It is a primary therapeutic goal for heart failure.
B. Higher levels of cyclic adenosine monophosphate in the vascular smooth muscle promote relaxation of the arterial and venous systems. This systemic vasodilation reduces both preload and afterload, facilitating more efficient ventricular emptying and improved peripheral perfusion. This action helps alleviate pulmonary vascular congestion.
E. Although primarily intended for inotropy, these agents often exert positive chronotropic effects as a secondary response to increased cyclic adenosine monophosphate in the sinoatrial node. This causes an increased heart rate, which must be monitored closely to prevent excessive myocardial oxygen consumption. It is a common physiological consequence of the drug.
Rationale for incorrect answers
C. Phosphodiesterase inhibitors generally increase or maintain heart rate rather than causing a decreased heart rate. A reduction in chronotropy is typically associated with beta-blockers or calcium channel blockers, which possess negative chronotropic properties. This medication class lacks the mechanism required to slow the cardiac conduction system.
D. Due to the potent vasodilatory effect on the systemic vasculature, these medications are more likely to cause hypotension than increased blood pressure. A significant drop in blood pressure is a known adverse effect that often requires dose adjustment or fluid boluses. Vasodilation decreases systemic vascular resistance, which naturally lowers the arterial tension.
Test-taking strategy
- Identify the drug category: Recognize that milrinone is an inodilator, a term used to describe drugs that both increase contraction and cause dilation.
- Recall the cellular mechanism: Focus on the role of cyclic AMP. In the heart, more cAMP equals more "power" (inotropy) and more "speed" (chronotropy).
- Evaluate vascular effects: In smooth muscle, more cAMP equals relaxation (vasodilation).
- Correlate mechanism with clinical signs:
- If the drug causes vasodilation, then increased blood pressure (Option 4) must be incorrect.
- If the drug is a stimulant (positive inotrope), then decreased heart rate (Option 3) is logically inconsistent with the drug's activating nature.
- Select multiple correct responses: Ensure all aspects of the "inodilator" definition are covered: Inotropy (Option 1) and Vasodilation (Option 2), plus the secondary Chronotropy (Option 5).
Take home points
- Phosphodiesterase inhibitors provide dual benefits by increasing the force of myocardial contraction and decreasing systemic vascular resistance.
- The nurse must continuously monitor the electrocardiogram for arrhythmias and tachycardia resulting from the positive chronotropic and inotropic effects.
- Blood pressure monitoring is critical during intravenous infusion as the vasodilatory properties frequently lead to significant hypotension.
- These medications are reserved for short-term, acute heart failure management due to the potential for increased long-term mortality with chronic use.
Comprehensive Questions
A nurse in a provider’s office is monitoring serum electrolytes for four older adult clients who take digoxin (Lanoxin) and furosemide (Lasix). Which of the following electrolyte values puts a client at risk for digoxin toxicity?
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to improve myocardial contractility and facilitate vagal tone. Toxicity risk increases when hypokalemia occurs, typically below 3.5 mEq/L, often due to concurrent loop diuretic therapy.
Rationale for correct answer
C. A serum potassium level of 3.4 mEq/L indicates hypokalemia, as the standard reference range is 3.5 to 5.0 mEq/L. Low extracellular potassium increases digoxin binding to the ATPase pump, significantly elevating the risk of digitalis toxicity. The nurse must recognize that even mild potassium deficits can trigger life-threatening ventricular dysrhythmias in the elderly. This value requires immediate clinical intervention and potential potassium supplementation to ensure patient safety.
Rationale for incorrect answers
A. A serum calcium level of 9.2 mg/dL is within the normal range of 9.0 to 10.5 mg/dL for an adult. While hypercalcemia can increase the risk of digoxin-induced arrhythmias, this specific value does not represent a metabolic risk. The client is not at an increased risk of toxicity based on this stable laboratory finding. Monitoring should continue, but no immediate corrective action is required for this parameter.
B. The calcium value of 10.3 mg/dL remains within the standard limits of the physiological reference range for serum calcium levels. Although it is near the upper threshold, it is not considered pathological hypercalcemia, which would be necessary to potentiate digitalis toxicity. The client’s cardiac sensitivity to digoxin is not adversely affected by this specific electrolyte concentration. Continuous surveillance is appropriate as part of routine geriatric care in a primary setting.
D. A potassium level of 4.8 mEq/L is a perfectly normal value, falling safely within the 3.5 to 5.0 mEq/L range. This level provides sufficient competition for the binding sites on the sodium-potassium pump, effectively maintaining a safe therapeutic drug profile. There is no evidence of hypokalemia that would predispose this client to the toxic manifestations of Lanoxin. The nurse should document this as a stable and therapeutic laboratory result.
Test-taking strategy
- Identify the core drug interaction: Recognize that the combination of digoxin and furosemide is high-risk because loop diuretics waste potassium.
- Establish reference ranges:
- Potassium: 3.5 to 5.0 mEq/L.
- Calcium: 9.0 to 10.5 mg/dL.
- Define digoxin toxicity risk: Recall that hypokalemia (low potassium), hypomagnesemia (low magnesium), and hypercalcemia (high calcium) are the primary triggers.
- Evaluate the choices:
- Choices 1, 2, and 4 are all within normal physiological limits.
- Choice 3 (3.4 mEq/L) is the only abnormal value and is specifically below the potassium threshold.
- Apply prioritization of risk: Select the value that represents a physiological deficit known to increase drug-receptor binding.
- Consider the patient population: Older adults have reduced renal clearance, making them even more susceptible to electrolyte-driven drug toxicity.
Take home points
- Hypokalemia is the most significant electrolyte imbalance increasing digoxin toxicity risk because it reduces competition for binding sites on the sodium-potassium ATPase pump.
- Loop diuretics like furosemide frequently cause potassium depletion, necessitating frequent serum electrolyte monitoring and often potassium-rich diets or supplements.
- Clinical signs of digoxin toxicity include gastrointestinal distress, bradycardia, and visual disturbances such as yellow-green halos or blurred vision.
- Therapeutic serum digoxin levels range from 0.5 to 2.0 ng/mL, but toxicity can occur at lower levels if significant electrolyte disturbances are present.
A nurse is administering a dopamine infusion at a moderate dose to a client who has severe heart failure.
Which of the following is an expected effect?
Explanation
Dopamine is a catecholamine vasopressor that stimulates beta-1 adrenergic receptors at moderate doses (2-10 mcg/kg/min) to provide positive inotropic support. It treats cardiogenic shock by increasing cardiac output while maintaining renal perfusion via dopaminergic activation at lower doses. Side effects include tachyarrhythmias and myocardial ischemia.
Rationale for correct answer
B. Moderate-dose dopamine stimulates beta-1 receptors in the myocardium. This activation increases myocardial contractility through enhanced calcium influx. Improved stroke volume directly addresses low output in heart failure patients. The resulting hemodynamic profile shows an increased cardiac index.
Rationale for incorrect answers
A. Dopamine typically causes tachycardia rather than a lowered heart rate due to beta-1 stimulation. It acts as a positive chronotropic agent by increasing the firing rate of the sinoatrial node. Decreasing heart rate is not a physiological effect of this catecholamine.
C. Beta-1 stimulation by dopamine actually enhances AV conduction rather than decreasing it. This dromotropic effect can predispose patients to supraventricular tachycardias or rapid ventricular responses. It does not provide the vagal-like slowing of conduction seen with other drugs.
D. At moderate doses, dopamine maintains or increases renal blood flow, not vasoconstriction. Alpha-1 mediated vasoconstriction typically occurs at high doses >10 mcg/kg/min. Moderate doses avoid the renal ischemia associated with pure alpha-adrenergic agonists like high-dose norepinephrine.
Test-taking strategy
- Identify the drug and dose: The question specifies a moderate dose of dopamine, which is the "sweet spot" for cardiac support.
- Apply receptor knowledge:
- Low dose (0.5-2 mcg/kg/min) targets dopaminergic receptors (renal dilation).
- Moderate dose (2-10 mcg/kg/min) targets beta-1 receptors (cardiac stimulation).
- High dose (>10 mcg/kg/min) targets alpha-1 receptors (systemic vasoconstriction).
- Rule out opposites: Choice 1 and 3 describe inhibitory effects (lowered rate/conduction), but catecholamines are excitatory stimulants.
- Distinguish dose-dependent effects: Choice 4 describes high-dose effects (vasoconstriction), whereas the question asks for moderate-dose effects.
- Match the condition: In heart failure, the therapeutic goal is to improve the pump, which aligns with increased contractility (Choice 2).
Take home points
- Moderate-dose dopamine (2-10 mcg/kg/min) primarily exerts positive inotropic and chronotropic effects via beta-1 receptor activation.
- Beta-1 stimulation increases the force of myocardial contraction and heart rate to improve overall cardiac output in failure states.
- High-dose dopamine (>10 mcg/kg/min) causes peripheral vasoconstriction by stimulating alpha-1 receptors, which increases systemic vascular resistance.
- Nurses must monitor for adverse effects such as chest pain, hypertension, and cardiac dysrhythmias during continuous intravenous titration.
A nurse is educating a client receiving hydralazine (Apresoline) The teaching plan should include which of the following points?
Explanation
Hydralazine is a direct-acting peripheral vasodilator that relaxes vascular smooth muscle, primarily in arterioles, to reduce systemic vascular resistance. It manages essential hypertension and hypertensive emergencies by decreasing afterload, though it may trigger compensatory tachycardia or a lupus-like syndrome.
Rationale for correct answer
D. Hydralazine causes significant arterial vasodilation, which frequently leads to orthostatic hypotension and dizziness upon changing positions. The client must be taught to rise slowly to prevent sudden drops in blood pressure and subsequent falls or syncope. This safety measure is essential due to the rapid reduction in peripheral resistance following drug administration. Compensatory reflex tachycardia may also occur, potentially increasing the risk of cardiac demand during positional changes.
Rationale for incorrect answers
A. Monthly urinalysis testing is not a standard requirement for clients specifically taking hydralazine therapy for hypertension. While the drug can cause a systemic lupus-like reaction, monitoring focuses on antinuclear antibody titers rather than renal excretion profiles. Urinary changes are not a common or specific side effect of this direct-acting vasodilator. The medication is primarily metabolized in the liver and does not necessitate routine monthly urine assessments.
B. Including specific citrus fruits or vegetables is a general healthy heart recommendation but is not specifically linked to the pharmacology of hydralazine. Unlike some other antihypertensives, this drug does not have a unique interaction with vitamin C or specific food groups mentioned in the choice. Clients should maintain a balanced diet, but this instruction is not a priority specific to hydralazine administration.
C. Decreasing potassium-rich food is an instruction typically reserved for clients taking potassium-sparing diuretics or angiotensin-converting enzyme inhibitors. Hydralazine does not affect the renin-angiotensin-aldosterone system or renal potassium handling, so hyperkalemia is not an expected side effect. Restricting potassium without clinical indication could lead to hypokalemia, which might exacerbate reflex tachycardia or other cardiac issues.
Test-taking strategy
- Identify the medication class: Recognize hydralazine as a direct-acting vasodilator, a class known for causing a rapid drop in blood pressure.
- Determine the primary side effect: Recall that vasodilation leads to decreased peripheral resistance, which commonly results in orthostatic hypotension.
- Prioritize safety and risk reduction:
- Choice 4 directly addresses the risk of falls and injury by providing a preventative behavioral intervention.
- Choices 1, 2, and 3 involve monitoring or dietary changes that do not align with the specific pharmacodynamics of hydralazine.
- Rule out class-specific diet restrictions:
- Potassium restriction (Choice 3) is for ACE inhibitors or ARBs, not vasodilators.
- Choice 2 is too general and lacks a specific scientific link to the drug's mechanism.
- Focus on the reflex response: Understand that the body's compensatory mechanisms for vasodilation make positional changes hazardous for the patient.
Take home points
- Hydralazine acts directly on arterial smooth muscle to cause vasodilation, effectively lowering blood pressure by reducing systemic vascular resistance.
- Orthostatic hypotension is a common adverse effect, necessitating patient education on slow position changes to prevent dizziness and accidental falls.
- Long-term use of high-dose hydralazine carries a risk for a drug-induced lupus-like syndrome, characterized by fever, joint pain, and positive antinuclear antibodies.
- Patients should be monitored for reflex tachycardia and sodium retention, which are common compensatory responses to the drug's potent vasodilatory actions.
The nurse is assessing a client who is receiving digoxin. The nurse monitors for findings that would indicate an increased possibility of toxicity, such as:
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to increase intracellular calcium, facilitating positive inotropy. It manages heart failure and tachyarrhythmias, but its therapeutic window is narrow, ranging from 0.5 to 2.0 ng/mL. Toxicity risk is significantly amplified by hypokalemia, as low potassium increases drug binding to myocardial receptors. Severe toxicity can manifest as life-threatening ventricular dysrhythmias or heart block.
Rationale for correct answer
C. A serum potassium level of 2.0 mEq/L indicates severe hypokalemia, which dramatically sensitizes the myocardium to digoxin. Potassium and digoxin compete for the same binding site on the ATPase pump enzyme. When extracellular potassium is significantly low, more digoxin molecules bind to the pump, leading to excessive intracellular calcium and toxicity. This specific electrolyte imbalance is the most common cause of digitalis-induced arrhythmias in clinical practice.
Rationale for incorrect answers
A. An apical pulse rate of 62 beats/min is within the normal range for an adult at rest. While digoxin has negative chronotropic effects, the nurse typically only withholds the medication if the heart rate drops below 60 beats/min. This heart rate does not suggest drug accumulation or an increased risk of toxic manifestations. Continuous monitoring is required, but this finding is currently hemodynamically stable.
B. The serum digoxin level of 1.5 ng/mL is within the accepted therapeutic range of 0.5 to 2.0 ng/mL. This concentration suggests that the medication is providing necessary inotropic support without reaching toxic thresholds in the bloodstream. A level of 1.5 ng/mL is generally considered safe and effective for treating congestive heart failure.
D. A serum calcium level of 9.9 mEq/L is a normal finding, as the reference range is typically 9.0 to 10.5 mEq/L. Although hypercalcemia can potentiate the effects of digoxin and lead to toxicity, this value does not represent an elevation. The client is not at an increased risk of cardiac glycoside complications based on this stable electrolyte profile.
Test-taking strategy
- Identify the core objective: The question asks for a finding that increases the possibility of toxicity, not necessarily a symptom of toxicity itself.
- Compare lab values to standard reference ranges:
- Potassium: 3.5 to 5.0 mEq/L (Choice 3 is significantly low).
- Digoxin: 0.5 to 2.0 ng/mL (Choice 2 is normal).
- Calcium: 9.0 to 10.5 mEq/L (Choice 4 is normal).
- Apply pharmacological principles: Recall that hypokalemia (low potassium) is the primary metabolic disturbance that triggers digoxin toxicity due to competitive binding at the cellular level.
- Analyze nursing assessment: An apical pulse of 62 (Choice 1) is above the standard hold parameter of 60, making it a normal finding.
- Use risk prioritization: When multiple choices involve electrolytes, select the one that has the most profound and direct impact on the medication's safety profile.
- Focus on preventative safety: Recognizing a low potassium level is a proactive nursing action to prevent toxicity before clinical symptoms emerge.
Take home points
- Hypokalemia significantly increases the risk of digoxin toxicity because potassium and digoxin compete for binding sites on the sodium-potassium ATPase pump.
- The therapeutic range for digoxin is narrow (0.5 to 2.0 ng/mL), necessitating frequent blood draws to ensure the patient remains within safe limits.
- Nurses must assess the apical pulse for 1 full minute before administration and withhold the dose if the heart rate is less than 60 beats/min.
- Other electrolyte imbalances that increase toxicity risk include hypomagnesemia and hypercalcemia, both of which can lead to life-threatening cardiac dysrhythmias.
A client is receiving an intravenous infusion of nesiritide (Natrecor). The nurse will look for which of the following adverse effects?
Explanation
Nesiritide is a recombinant B-type natriuretic peptide that facilitates cyclic guanosine monophosphate mediated vasodilation to reduce pulmonary capillary wedge pressure. It manages decompensated heart failure by inducing natriuresis, though it can trigger significant dysrhythmia or dose-dependent hypotension.
Rationale for correct answer
A. Nesiritide administration is associated with various cardiac conduction disturbances, primarily including the development of dysrhythmia or palpitations during the infusion. This recombinant peptide can increase the incidence of ventricular tachycardia in patients with existing myocardial instability. The nurse must monitor the electrocardiogram continuously to detect premature ventricular contractions or other proarrhythmic events. This adverse effect is a documented risk during the acute management of heart failure. Proarrhythmic effects necessitate immediate clinical evaluation and potentially titrating the infusion rate downward to maintain patient safety.
Rationale for incorrect answers
B. Nesiritide actually promotes renal excretion of sodium and water, but it is not typically associated with the development of proteinuria. Protein in the urine usually indicates glomerular damage or chronic kidney disease rather than an acute reaction to natriuretic peptides. The drug focuses on hemodynamic unloading rather than altering the permeability of the glomerular membrane. Therefore, this finding is not an expected adverse effect of this specific therapy.
C. There is no established scientific evidence linking the administration of intravenous nesiritide to the development of hyperglycemia. Glucose metabolism is not affected by the cyclic guanosine monophosphate pathways targeted by this recombinant peptide medication. Significant elevations in blood glucose are more commonly associated with corticosteroid therapy or diabetic decompensation rather than vasodilators. The nurse should prioritize monitoring blood pressure over routine glucose checks for this medication.
D. The primary pharmacological effect of nesiritide is potent vasodilation, which results in hypotension rather than hypertension. The drug reduces systemic vascular resistance to unload the failing heart, frequently causing a significant drop in mean arterial pressure. If a client develops high blood pressure, it is likely due to an underlying condition or a different pharmacological agent. Hypertension would represent a failure of the medication's intended therapeutic action.
Test-taking strategy
- Identify the drug class: Recognize nesiritide as a human B-type natriuretic peptide (hBNP) used for acute heart failure.
- Identify the intended effect: Understand that "natriuretic" and "peptide" imply vasodilation and fluid excretion, which lowers blood pressure.
- Analyze cardiac risks:
- Recall that vasoactive drugs used in acute care frequently affect cardiac electrophysiology.
- Choice 1 (Dysrhythmia) is a common risk for many inotropic and vasodilatory agents used in heart failure management.
- Evaluate physiological logic:
- Rule out Choice 4 because the drug is a vasodilator (causes low, not high, pressure).
- Rule out Choice 3 because BNP doesn't interfere with insulin or glucose signaling.
- Rule out Choice 2 because while it affects the kidney, it does not typically cause protein leakage.
- Focus on safety: Prioritize the answer that relates to acute hemodynamic or rhythmic instability, which is most relevant in a critical care setting.
Take home points
- Nesiritide is a recombinant B-type natriuretic peptide that reduces preload and afterload by stimulating cyclic guanosine monophosphate in vascular smooth muscle.
- The most common and significant adverse effect is dose-related hypotension, requiring frequent blood pressure monitoring throughout the duration of the infusion.
- Nesiritide can be proarrhythmic, increasing the risk of ventricular dysrhythmias, which necessitates continuous telemetry or electrocardiogram observation.
- Nursing care for patients on nesiritide involves strict intake and output monitoring to evaluate the diuretic and natriuretic effectiveness of the treatment.
The medication order for a client who receives nutrition via a feeding tube reads: “Give digoxin 0.125 mg per feeding tube once every morning.” The medication is available in a liquid form 50 mcg/mL. How many milliliters will the nurse give for each dose?
Explanation
Digoxin is a cardiac glycoside that exerts positive inotropic effects by inhibiting the Na+/K+-ATPase pump. This increases intracellular calcium, facilitating myocardial contraction in patients with congestive heart failure. Pharmacological dosing requires precise milligram-microgram conversions to avoid life-threatening digitalis toxicity. The therapeutic reference range is 0.5 to 2.0 ng/mL, and the drug is contraindicated in ventricular fibrillation or obstructive cardiomyopathy.
Rationale for correct answer
The nurse must first perform a metric conversion where 0.125 mg is multiplied by 1000.
This is equal to 125 mcg.
Using the standard formula, the desired dose of 125 mcg is divided by the available concentration of 50 mcg.
This calculation yields a volume of exactly 2.5 mL to be administered.
Test-taking strategy
- Identify the drug and units: The medication is digoxin, and the order is in mg, while the supply is in mcg.
- Convert to a common unit:
- Always convert to the unit available on the label to simplify the final step.
- 0.125 mg × 1000 = 125 mcg.
- Apply the dosage formula:
- Use (Desired / Have) × Quantity.
- (125 mcg / 50 mcg) × 1 mL = 2.5 mL.
- Verify the logic:
- If 1 mL contains 50 mcg, then 2 mL contains 100 mcg.
- The dose is 125 mcg, so the answer must be more than 2 mL but less than 3 mL.
- 2.5 mL is the only mathematically logical result based on this estimation.
- Safety check: Digoxin has a narrow therapeutic index. Any calculated volume that seems unusually large (like 25 mL) should be immediately questioned.
Take home points
- Accurate conversion between milligrams and micrograms is the most critical step in calculating cardiac glycoside dosages.
- Standardized liquid concentrations of digoxin are often measured in micrograms to ensure high precision in dose delivery.
- Doubling-checking the decimal point placement is essential, as a single-place error can result in a ten-fold overdose.
- Always use a calibrated oral syringe for feeding tube administration to ensure the exact delivery of small-volume medications.
Scenario
An elderly client with known heart failure, who has previously been stable with lisinopril and metoprolol XL, comes into the clinic with an increase in weight and peripheral edema extending halfway up the left calf.
The nurse recognizes the primary actions of the drugs used to for heart failure. Indicate with an X the effect each drug classification will have.
Explanation
Heart failure therapy utilizes neurohormonal modulation to counteract pathological remodeling and fluid retention. Pharmacological agents target the renin-angiotensin-aldosterone system or sympathetic nervous system to optimize hemodynamic parameters. These interventions decrease the myocardial workload by manipulating vascular tone and salt excretion. Standard care involves maintaining serum potassium between 3.5 and 5.0 mEq/L and monitoring ejection fraction percentages.
Rationale for correct answer
ACE Inhibitors. These agents block the conversion of angiotensin 1 to angiotensin 2, a potent vasoconstrictor. This leads to decreased peripheral resistance and systemic arterial pressure. By inhibiting aldosterone secretion, they also reduce sodium retention, effectively decreasing both preload and afterload.
ARBs. Angiotensin receptor blockers prevent the binding of angiotensin 2 to its specific vascular receptors. This causes systemic vasodilation and a significant afterload reduction. Like ACE inhibitors, they mitigate fluid volume through aldosterone suppression, thereby decreasing cardiac preload effectively.
Beta Blockers. These medications competitively antagonize beta-1 adrenergic receptors located on the myocardium. This action inhibits the sympathetic response, specifically reducing catecholamine-induced damage. The primary clinical result is a slower rate, which increases diastolic filling time and efficiency.
ARB/Neprilysin. This combination blocks the angiotensin receptor while simultaneously inhibiting the enzyme that degrades beneficial vasodilator proteins. This dual action significantly increases peptides like BNP that promote natriuresis. It provides superior vasodilation and volume reduction compared to standard mono-therapy.
Test-taking strategy
- Categorize by mechanism: Group ACE inhibitors, ARBs, and ARNIs together as they all target the RAAS and primarily affect vascular resistance.
- Identify unique actions:
- Only Beta Blockers significantly impact heart rate and sympathetic nerve stimulation.
- Only ARNI (Entresto) impacts neprilysin to increase natriuretic peptides.
- Match symptoms to therapy:
- Peripheral edema and weight gain require preload reduction (ACE/ARB/ARNI).
- High systemic pressure requires afterload reduction (ACE/ARB/ARNI).
- Rule out cross-over errors: Do not attribute "increased natriuretic peptides" to standard ACE inhibitors, as this is a distractor specific to newer combination therapies.
- Focus on the "X" requirements: Ensure that for each drug, every correct physiological effect is marked, as these drugs have multi-modal impacts on the cardiovascular system.
Take home points
- ACE inhibitors and ARBs are foundational for heart failure because they reduce both the pressure the heart pumps against and the fluid volume returning to it.
- Beta blockers are vital for long-term survival by shielding the heart from chronic sympathetic overstimulation and reducing oxygen demand.
- Sacubitril/valsartan (ARNI) is unique because it preserves natriuretic peptides that help the body naturally excrete sodium and dilate vessels.
- Monitoring for hypotension and hyperkalemia is a priority for all RAAS-modifying drugs, while heart rate must be assessed for beta blockers.
Scenario
An elderly client with known heart failure, who has previously been stable with lisinopril and metoprolol XL, comes into the clinic with an increase in weight and peripheral edema extending halfway up the left calf.
It was decided that the client in the scenario start on digoxin. The nurse is providing instructions on what to watch for, to detect developing digitalis toxicity. Which of the following signs and symptoms should be included? Select all that apply
Explanation
Digoxin is a cardiac glycoside that inhibits the sodium-potassium ATPase pump to induce positive inotropy. It increases intracellular calcium concentrations while enhancing vagal tone, thereby slowing conduction through the atrioventricular node. Excessive drug accumulation leads to digitalis toxicity, which frequently manifests as gastrointestinal distress, neurological disturbances, and life-threatening cardiac arrhythmias.
Rationale for correct answers
A. Digoxin increases parasympathetic activity, leading to bradycardia via the inhibition of the sinoatrial node and atrioventricular node. Toxicity typically presents with a heart rate < 60 beats/min due to excessive vagal stimulation. The nurse must instruct the client to assess their radial pulse daily before drug administration. Significant slowing of the heart rate is a primary indicator of toxic accumulation.
B. Gastrointestinal symptoms are often the earliest clinical markers of toxicity, with anorexia being a classic early warning sign. The drug triggers the chemoreceptor trigger zone in the medulla, leading to a profound loss of appetite and nausea. Clients should report any sudden changes in nutritional intake or persistent abdominal discomfort. Early recognition of these symptoms can prevent progress to more severe cardiac complications.
C. Neurological and ophthalmic manifestations of digitalis toxicity include blurred vision or the perception of yellow-green halos. These disturbances occur due to the inhibition of ATPase in the retina, altering normal visual signal processing. The client may also report seeing spots or experiencing generalized hazy vision during daily activities. Visual changes are highly specific indicators that the serum digoxin level has exceeded the therapeutic threshold.
Rationale for incorrect answers
D. Digoxin toxicity is not associated with hypertension, as the drug does not significantly influence systemic vascular resistance. While it improves cardiac output, its toxic effects are primarily related to arrhythmogenesis and gastrointestinal irritation. Blood pressure may actually decrease if the client develops severe bradycardia or high-degree heart block. Monitoring should focus on heart rate and rhythm rather than elevations in pressure.
E. There is no established scientific link between cardiac glycoside toxicity and hearing loss or auditory dysfunction. Digoxin primarily affects the visual system and the central nervous system but does not target the cochlea or auditory nerves. Symptoms such as tinnitus or deafness are more characteristic of ototoxic medications like aminoglycosides or loop diuretics. The nurse should prioritize screening for visual disturbances instead.
Test-taking strategy
- Identify the pathophysiology of toxicity: Recognize that digoxin has a very narrow therapeutic index (0.5 to 2.0 ng/mL) and affects multiple organ systems.
- Categorize clinical manifestations:
- Cardiac: Bradycardia and heart blocks (Choice 1).
- Gastrointestinal: Nausea, vomiting, and anorexia (Choice 2).
- Visual/Neurological: Halos, blurred vision, and confusion (Choice 3).
- Rule out unrelated symptoms:
- Digoxin is used for heart failure and rate control; it is not an antihypertensive, so hypertension (Choice 4) is incorrect.
- Ototoxicity (Choice 5) is a side effect of drugs like furosemide, not digoxin.
- Apply safety prioritization: Prioritize signs that the client can easily monitor at home, such as pulse rate and appetite changes.
- Use the select all that apply technique: Evaluate each choice independently against the known toxic profile of the cardiac glycoside class.
Take home points
- Digoxin toxicity presents with a triad of gastrointestinal, visual, and cardiac symptoms, requiring immediate medical evaluation.
- The most frequent early signs of toxicity are anorexia, nausea, and bradycardia, which may precede life-threatening arrhythmias.
- Therapeutic serum digoxin levels must be maintained between 0.5 and 2.0 ng/mL, with toxicity frequently occurring when levels exceed 2.4 ng/mL.
- Clients must be educated to take their pulse for 1 full minute and withhold the dose if the heart rate is less than 60 beats/min.
The nurse is teaching a client about the signs and symptoms of cardiac glycoside toxicity. The nurse should alert the client to watch for which of the following? Select all that apply
Explanation
Cardiac glycosides like digoxin inhibit the Na+/K+-ATPase pump, increasing intracellular calcium to enhance myocardial contractility. This narrow therapeutic index drug requires serum levels between 0.5 to 2.0 ng/mL. Toxicity precipitates life-threatening dysrhythmias, gastrointestinal distress, and profound neurological or sensory disturbances. Hypokalemia significantly increases the risk of digitalis toxicity.
Rationale for correct answers
A. Excessive levels of digoxin interfere with cerebral electrical activity and systemic hemodynamics. This neurotoxicity often manifests as significant dizziness or lightheadedness in the affected patient. It reflects the drug's impact on the central nervous system. The nurse must identify this as an early warning sign.
B. Digoxin toxicity frequently affects the cones of the retina and the optic nerve. Patients classically report visual changes such as xanthopsia or seeing yellow-green halos around lights. This is a hallmark sign of advanced glycoside accumulation. Immediate clinical evaluation and serum level checks are mandatory.
C. Neurological irritation from toxic levels of cardiac glycosides can trigger persistent discomfort. Severe headaches are documented as a common symptomatic manifestation of digitalis-induced neuralgia. This symptom often precedes more dangerous cardiac conduction blocks. Monitoring for these neurological changes is essential for safety.
Rationale for incorrect answers
D. Digoxin is used to improve cardiac output, which should theoretically support kidney perfusion and maintain urination. However, increased urine output is a therapeutic goal of diuretics, not a sign of glycoside toxicity. Toxicity is more likely to cause nausea, vomiting, or profound bradycardia. It does not manifest as a sudden diuresis.
E. The presence of melena or dark stools typically indicates upper gastrointestinal bleeding or iron supplementation. Digoxin toxicity causes anorexia and abdominal pain but does not cause gastrointestinal hemorrhage. This finding would suggest a different pathology entirely, such as a peptic ulcer. It is not associated with cardiac glycoside levels.
Test-taking strategy
- Identify the drug class and its risks: Digoxin has a very narrow therapeutic window, meaning the difference between a helpful dose and a toxic dose is small.
- Recall the "big three" of digoxin toxicity:
- Gastrointestinal: Nausea, vomiting, anorexia.
- Cardiac: Bradycardia, heart blocks, PVCs.
- Neurological/Sensory: Confusion, headaches, dizziness, and the classic yellow-green halos (xanthopsia).
- Evaluate the options against these categories:
- Options 1, 2, and 3 fit perfectly into the neurological and sensory categories.
- Rule out unrelated physiological effects:
- Increased urine output (Option 4) is the goal of diuretics, which are often taken with digoxin but have a different toxicity profile.
- Dark stools (Option 5) is a sign of bleeding or medication like iron/bismuth, not heart medication toxicity.
- Prioritize the "hallmark" sign: If you see "halos" or "yellow vision" in a digoxin question, it is almost always related to toxicity.
Take home points
- Digoxin toxicity often presents first with gastrointestinal symptoms like anorexia and nausea before progressing to neurological and cardiac signs.
- Visual disturbances, specifically seeing yellow-green halos around lights, are classic indicators of digoxin overdosage.
- Patients must be educated to report new-onset dizziness or headaches, as these can signal impending cardiac arrhythmias.
- Low serum potassium levels increase the risk of digoxin toxicity, so patients must maintain stable electrolyte balances during therapy.
An older adult client has been discharged following treatment for heart failure, and will be taking a loop diuretic. Which instructions from the nurse are appropriate? Select all that apply
Explanation
Loop diuretics inhibit the Na+/K+/2Cl- symporter within the thick ascending limb of the loop of Henle, inducing potent natriuresis and subsequent fluid volume reduction. This class addresses congestive heart failure and pulmonary edema but risks significant hypokalemia, prerenal azotemia, and ototoxicity. Serum potassium must remain between 3.5 and 5.0 mEq/L. Contraindications include anuria or severe sulfonamide hypersensitivity.
Rationale for correct answers
A. Establishing a consistent administration schedule ensures stable pharmacokinetic levels and optimizes therapeutic outcomes for the patient. Taking the medication in the morning specifically prevents nocturia, which can disrupt sleep and increase fall risks. This routine promotes pharmacological adherence in chronic management.
C. Loop diuretics significantly reduce intravascular volume and lower systemic venous pressure, which can lead to orthostatic hypotension. Rising slowly allows time for baroreceptor compensation to prevent syncope and subsequent injuries. This instruction is vital for ensuring the safety of older adult patients.
F. Muscle weakness is a primary clinical manifestation of hypokalemia, while dizziness often indicates significant dehydration or hypotension. These symptoms signal dangerous electrolyte imbalances or hemodynamic instability that require immediate medical evaluation. Reporting these findings early prevents lethal cardiac arrhythmias.
Rationale for incorrect answers
B. Diuretics for chronic heart failure are intended for consistent daily use to maintain a stable euvolemic state. Taking the medication only when edema is visible allows for dangerous fluid accumulation in the pulmonary vasculature. Effective management requires proactive dosing rather than reactive, symptom-based administration.
D. Prescribing a high fluid intake of 8 glasses daily directly contradicts the therapeutic goal of treating heart failure. Excessive water consumption can exacerbate volume overload and worsen symptoms like dyspnea and orthopnea. Fluid intake must be carefully balanced or restricted based on the patient's renal and cardiac status.
E. Loop diuretics are non-potassium-sparing agents that promote the renal excretion of potassium into the tubular lumen. Patients actually require increased intake of potassium-rich foods to offset the drug-induced losses and prevent hypokalemia. Advising the avoidance of potassium is incorrect and potentially life-threatening for this medication class.
Test-taking strategy
- Identify the physiological priority: The client is an older adult with heart failure. The primary goals are managing volume while preventing injury from drug-induced hypotension.
- Apply knowledge of diuretic classes: Distinguish between loop diuretics and potassium-sparing diuretics.
- Since loop diuretics waste potassium, any choice suggesting the avoidance of potassium (option 5) is scientifically incorrect.
- Prioritize patient safety and stability:
- Consistent timing (option 1) ensures the drug works throughout the day and protects sleep.
- Postural safety (option 3) addresses the high risk of orthostatic changes.
- Symptom reporting (option 6) focuses on the most dangerous metabolic and hemodynamic complications.
- Evaluate the disease process: Heart failure often requires fluid restriction, not high-volume hydration (option 4). Furthermore, chronic medications are rarely used on a "PRN" or as-needed basis for physical swelling (option 2) unless specified for minor symptoms, as consistent dosing is required to prevent pulmonary congestion.
Take home points
- Loop diuretics like furosemide or bumetanide require patients to rise slowly to prevent syncope associated with orthostatic hypotension.
- Daily morning administration is recommended to achieve peak diuresis during the day and prevent nocturnal sleep disruption.
- Clinical signs of hypokalemia, such as muscle weakness or cramping, must be reported immediately to prevent cardiac conductivity issues.
- Patients on loop diuretics should generally increase their consumption of potassium-rich foods to maintain serum levels ≥ 3.5 mEq/L.
A client is taking hydrochlorothiazide (HCTZ) 50 mg/ day and digoxin (Lanoxin) 0.25 mg/day. The nurse plans to monitor the client for which potential electrolyte imbalance?
Explanation
Hydrochlorothiazide is a thiazide diuretic that targets the distal convoluted tubule by inhibiting sodium-chloride symporters, thereby promoting potassium excretion and diuresis. It is indicated for hypertension and congestive heart failure management but frequently precipitates metabolic complications. This drug-induced electrolyte imbalance increases myocardial sensitivity to cardiac glycosides, potentially leading to lethal arrhythmias.
Rationale for correct answer
B. Thiazides increase sodium delivery to the late distal tubule, which accelerates the secretion of potassium into the urine. This process leads to hypokalemia, defined as serum potassium levels < 3.5 mEq/L. Low potassium is extremely dangerous for clients taking digoxin because it enhances drug binding to the sodium-potassium ATPase pump. This interaction significantly increases the risk of life-threatening digitalis toxicity.
Rationale for incorrect answers
A. Thiazide diuretics are known to decrease the renal excretion of calcium, which typically results in hypercalcemia rather than low levels. They enhance calcium reabsorption in the distal tubule, making them useful for patients with osteoporosis or kidney stones. Therefore, hypocalcemia is not a characteristic metabolic finding associated with the administration of hydrochlorothiazide therapy.
C. Thiazide and loop diuretics are definitively potassium-wasting agents that facilitate the loss of this cation. Hyperkalemia is only associated with potassium-sparing diuretics, such as spironolactone, or the use of ACE inhibitors. This result would be the physiological opposite of the expected effect of a 50 mg daily dose of hydrochlorothiazide.
D. Thiazide diuretics generally increase the renal excretion of magnesium, which leads to a state of hypomagnesemia. An elevated level, or hypermagnesemia, is rare and usually only occurs in the setting of advanced renal failure or excessive intake of antacids. Like potassium, low magnesium levels actually potentiate the toxic effects of digoxin on the cardiac conduction system.
Test-taking strategy
- Identify the drug interaction: The question pairs a thiazide diuretic (HCTZ) with digoxin. This is a classic "must-know" pharmacological combination in nursing.
- Recall the mechanism of HCTZ: Thiazides are non-potassium-sparing. Their primary metabolic side effect is the depletion of potassium, sodium, and magnesium.
- Prioritize the most dangerous imbalance: While several electrolytes change, hypokalemia is the most clinically significant because of its direct relationship with digoxin toxicity.
- Digoxin competes with potassium for binding sites on the Na+/K+ ATPase pump.
- Low potassium means more available binding sites for digoxin, leading to toxic levels even with standard doses.
- Eliminate based on "Hyper" vs "Hypo":
- Thiazides waste potassium, so any "hyper" potassium option (option 3) is incorrect.
- Thiazides "save" calcium, so a "hypo" calcium option (option 1) is incorrect.
- Thiazides waste magnesium, making a "hyper" magnesium option (option 4) incorrect.
Take home points
- Hydrochlorothiazide frequently causes hypokalemia, which is a major risk factor for the development of digoxin toxicity.
- Clients on this combination must be taught to recognize signs of digitalis toxicity, such as yellow-green halos, nausea, and bradycardia.
- Serum potassium levels should be strictly monitored and maintained within the range of 3.5 to 5.0 mEq/L to ensure cardiac safety.
- High-potassium foods or oral potassium supplements are often required when taking thiazide diuretics to offset mandatory renal losses.
A nurse is educating a heart failure client about diuretics. Which adverse reaction associated with ACE inhibitors is common and can lead to disruption of therapy?
Explanation
Angiotensin-converting enzyme inhibitors prevent the conversion of angiotensin 1 to angiotensin 2, causing systemic vasodilation and decreased aldosterone secretion. This mechanism results in increased bradykinin levels within the pulmonary vasculature, often precipitating a persistent, non-productive inflammation of the airways. These agents treat hypertension and heart failure but carry risks of angioedema and hyperkalemia.
Rationale for correct answer
B. Inhibition of angiotensin-converting enzyme prevents the breakdown of bradykinin and substance P in the lung tissue. This accumulation triggers a persistent, dry, hacking cough in approximately 5 to 20 percent of patients. Because this effect is often refractory to antitussive medications, it frequently necessitates the permanent discontinuation of therapy. It is the most common reason for switching to an angiotensin receptor blocker.
Rationale for incorrect answers
A. ACE inhibitors are generally associated with gastrointestinal disturbances like nausea or diarrhea rather than decreased bowel motility. Constipation is not a documented or clinically significant side effect of this medication class in human trials. If a patient experiences infrequent stools, other factors or concurrent medications should be investigated
C. While certain antihypertensive classes like beta-blockers or diuretics can impact reproductive health, ACE inhibitors typically do not cause sexual dysfunction. These drugs are often selected as alternatives when a patient experiences such issues on other therapies. They maintain relatively neutral effects on hemodynamics related to sexual performance.
D. ACE inhibitors primarily cause a reduction in systemic vascular resistance without a significant reflexive increase in heart rate. Significant tachycardia is not a standard reaction and might actually indicate an underlying compensatory mechanism for hypotension. Patients are more likely to experience dizziness or orthostatic changes during the initiation phase.
Test-taking strategy
- Identify the specific drug class: Focus on ACE inhibitors (often ending in -pril, such as lisinopril).
- Recall class-specific adverse effects: Distinguish between general side effects and those unique to this mechanism of action.
- Connect the mechanism to the symptom: Recall that ACE is the same enzyme that degrades bradykinin. Inhibiting the enzyme means bradykinin stays in the lungs, causing irritation.
- Evaluate the impact on compliance: The question asks for a "common" reaction that leads to "disruption of therapy." While many side effects exist, the dry cough is the most frequent reason patients cannot tolerate the drug.
- Rule out common distractors:
- Sexual dysfunction is a common reason for stopping beta-blockers.
- Constipation is a common side effect of calcium channel blockers (like verapamil).
- Tachycardia is a reflexive response often seen with hydralazine or other pure vasodilators, but not ACE inhibitors.
Take home points
- The persistent dry cough associated with ACE inhibitors is caused by the accumulation of bradykinin and typically resolves within days of stopping the medication.
- Angioedema is a rare but life-threatening adverse effect characterized by swelling of the tongue and airway that requires immediate emergency intervention.
- Patients on ACE inhibitors must be monitored for hyperkalemia because the suppression of aldosterone leads to decreased renal potassium excretion.
- If a cough becomes intolerable, the healthcare provider will typically transition the patient to an angiotensin receptor blocker, which does not affect bradykinin levels.
The nurse is teaching a client with chronic heart failure about spironolactone therapy. Which client statement indicates correct understanding?
Explanation
Spironolactone is a steroid-based aldosterone antagonist that competitively inhibits mineralocorticoid receptors in the distal nephron. This potassium-sparing diuretic prevents sodium-potassium exchange, effectively reducing myocardial fibrosis in heart failure. Chronic use can cause gynecomastia or hyperkalemia. It is specifically contraindicated in patients with anuria, acute renal insufficiency characterized by a creatinine clearance < 30 mL/min, or baseline serum potassium levels > 5.0 mEq/L.
Rationale for correct answer
B. Spironolactone is a non-selective antagonist that also binds to progesterone and androgen receptors, leading to significant endocrine side-effects. Men may experience painful gynecomastia, while women might report menstrual irregularities or breast tenderness. Identifying these symptoms indicates that the client understands the specific hormonal adverse effects associated with long-term mineralocorticoid receptor blockade.
Rationale for incorrect answers
A. Because spironolactone is a potassium-sparing agent, it prevents the renal excretion of potassium ions. Increasing the intake of potassium-rich foods or using salt substitutes can lead to lethal hyperkalemia. The client must be taught to maintain a consistent, moderate intake rather than increasing it. This statement reflects a dangerous knowledge deficit that could lead to cardiac arrest.
C. Unlike loop diuretics such as furosemide, spironolactone has relatively weak natriuretic properties and a slow onset of action. It is used primarily for its survival benefits and neurohormonal inhibition rather than rapid fluid mobilization. It will not provide immediate relief for acute pulmonary congestion or dyspnea. This incorrectly identifies the drug as a rescue medication for acute distress.
D. There is no pharmacological requirement or evidence-based clinical guideline suggesting spironolactone must be taken with a high-protein meal. While taking it with food may slightly increase bioavailability, the specific protein content is irrelevant to its therapeutic efficacy. The instruction is medically unnecessary and does not contribute to medication safety.
Test-taking strategy
- Identify the medication class: Spironolactone is a potassium-sparing diuretic and an aldosterone antagonist.
- Recall unique side effects: Beyond electrolyte shifts, think about the structural similarity of spironolactone to steroid hormones.
- This makes hormonal effects like gynecomastia a "must-know" for this specific drug.
- Evaluate safety risks:
- Since it spares potassium, rule out any answer suggesting potassium supplementation (Option 1).
- Differentiate between "weak" and "strong" diuretics; spironolactone is for long-term management, not rapid relief (Rule out Option 3).
- Eliminate distractors: Option 4 is a common type of distractor that adds specific, irrelevant dietary requirements to sound "scientific."
- Select the most specific answer: Option 2 is a highly specific, well-documented adverse effect that is unique to this class compared to loop or thiazide diuretics.
Take home points
- Spironolactone provides mortality benefits in heart failure by inhibiting aldosterone-mediated cardiac remodeling and fibrosis.
- Patients must be strictly monitored for hyperkalemia, especially if they are also taking ACE inhibitors or ARBs.
- Endocrine side effects like gynecomastia in men and breast tenderness in women are common reasons for treatment non-compliance.
- This medication is not a first-line agent for acute volume overload and should be viewed as a chronic disease-modifying therapy.
A client with heart failure asks why they are prescribed hydralazine and isosorbide dinitrate together. What is the best response by the nurse?
Explanation
The fixed-dose combination of hydralazine and isosorbide dinitrate acts through complementary vasodilation mechanisms to optimize cardiac hemodynamics. Hydralazine primarily facilitates arteriolar dilation, reducing systemic vascular resistance and afterload, while isosorbide dinitrate promotes venous dilation, decreasing preload. This synergistic approach effectively manages heart failure by mitigating the high ventricular wall stress. It is particularly indicated for patients with reduced ejection fraction who remain symptomatic despite standard therapy or as a first-line option in specific ethnic populations.
Rationale for correct answer
D. Hydralazine reduces the resistance against which the left ventricle must pump, while isosorbide dinitrate decreases the volume returning to the heart. This combined reduction in afterload and preload significantly lowers the metabolic oxygen demand of the myocardium. By decreasing the cardiac workload, the heart can pump more efficiently without overexertion. This accurately explains the hemodynamic benefits of dual vasodilator therapy to the client.
Rationale for incorrect answers
A. Neither hydralazine nor isosorbide dinitrate possesses biochemical properties that interfere with the HMG-CoA reductase pathway or cholesterol absorption. Managing cholesterol levels requires statins or other lipid-lowering agents, not systemic vasodilators. This explanation is scientifically inaccurate as it confuses cardiovascular hemodynamic support with lipid metabolism.
B. Vasodilators facilitate cardiac emptying but do not directly promote the renal excretion of sodium and water like diuretic therapy. While they may decrease the severity of congestion, they cannot replace the natriuretic effect of drugs like furosemide. Patients with fluid overload typically require both classes to manage the total volume status effectively.
C. Hydralazine and isosorbide dinitrate do not interact with the renal tubules or the mineralocorticoid receptors to influence electrolyte secretion. They are not potassium-sparing agents and have no clinical impact on the potassium wasting often induced by loop diuretics. Protecting against potassium loss requires potassium supplements or specific diuretics like spironolactone.
Test-taking strategy
- Identify drug mechanisms: Recall that hydralazine is an arterial vasodilator (afterload) and nitrates are venous vasodilators (preload).
- Apply heart failure principles: The goal of treating heart failure is to make it easier for the weak heart to pump.
- Analyze hemodynamic effects:
- If arteries are dilated, the heart pumps against less pressure.
- If veins are dilated, the heart receives less volume.
- Both lead to a direct reduction in cardiac workload (Option 4).
- Rule out non-related categories:
- Lipids/cholesterol are managed by different classes (Rule out Option 1).
- Diuretics/electrolytes are renal-acting, whereas these are vascular-acting (Rule out Options 2 and 3).
- Prioritize the "Best" response: Select the answer that addresses the core physiological benefit for a heart failure patient.
Take home points
- The combination of hydralazine and isosorbide dinitrate is a powerful strategy to reduce both preload and afterload in heart failure.
- This drug combination is specifically proven to improve survival and reduce hospitalizations in patients with heart failure with reduced ejection fraction.
- Nurses must monitor for reflex tachycardia and severe headache, which are common side effects due to the potent vasodilatory effects.
- Unlike ACE inhibitors or ARBs, this combination does not significantly impact renal function or serum potassium levels.
The nurse is reviewing the medications that have been ordered for a client for whom a loop diuretic has just been prescribed. The loop diuretic may have a possible interaction with which of the following? Select all that apply
Explanation
Loop diuretics reversibly inhibit Na+/K+/2Cl- symporters within the thick ascending limb of Henle to treat pulmonary edema. These high-ceiling agents induce potent natriuresis but significantly alter electrolyte homeostasis and glucose metabolism. Adverse effects include ototoxicity, profound hypokalemia (potassium < 3.5 mEq/L), and hyperuricemia. Contraindications include anuria and sulfonamide hypersensitivity.
Rationale for correct answers
B. Loop diuretics can interfere with pancreatic insulin release and diminish peripheral insulin sensitivity in target tissues. This pharmacological interaction often leads to elevated blood glucose levels, effectively antagonizing the efficacy of antidiabetic drugs. Consequently, patients may require increased dosages of hypoglycemic agents to maintain glycemic control. Continuous monitoring of serum glucose is mandatory.
D. Nonsteroidal anti-inflammatory drugs inhibit the synthesis of renal prostaglandins that are vital for maintaining renal blood flow. This inhibition blunts the vasodilatory response required for loop diuretics to function effectively, leading to decreased diuresis. These NSAIDs cause sodium retention, which directly opposes the therapeutic goal of fluid removal. This can exacerbate congestive heart failure symptoms.
E. Both loop diuretics and corticosteroids promote the renal excretion of potassium, leading to an additive risk of depletion. Concurrent administration significantly predisposes the patient to severe, life-threatening hypokalemia, which may trigger cardiac arrhythmias. Glucocorticoids also contribute to sodium retention and hyperglycemia, further complicating the diuretic's metabolic profile. Serum electrolyte surveillance must be intensified during co-administration.
Rationale for incorrect answers
A. There is no documented significant pharmacological interaction between loop diuretics and vitamin D supplementation. Vitamin D primarily regulates calcium and phosphorus absorption in the intestine and bone mineralization processes. While diuretics affect renal calcium handling, they do not interfere with the metabolic activation of this vitamin. It is considered safe to administer these concurrently in standard clinical practice.
C. Most penicillins are excreted via the kidneys through tubular secretion, but they do not share a common metabolic pathway with diuretics. There is no evidence that loop diuretics alter the antimicrobial efficacy or increase the systemic toxicity of penicillin-class antibiotics. These medications are routinely used together without requiring specific dosage adjustments for interaction. Clinical focus remains on standard allergy assessment only.
F. While diuretics can reduce plasma volume and theoretically concentrate clotting factors, they do not have a clinically significant interaction with warfarin. The anticoagulant effect of warfarin depends on vitamin K epoxide reductase inhibition, a pathway unaffected by loop diuretics. Regular monitoring of the International Normalized Ratio remains the standard for safety. No direct pharmacokinetic interference between these two drugs has been established.
Test-taking strategy
- Identify the drug class: Loop diuretics are potent fluid-removers that significantly impact electrolytes and metabolic pathways.
- Recall the "Hyper" and "Hypo" effects:
- Loop diuretics cause hyperglycemia. This logic leads directly to an interaction with antidiabetic drugs (Option 2).
- Loop diuretics cause hypokalemia. Combining them with other potassium-wasting drugs like corticosteroids (Option 5) is a major safety risk.
- Apply the "Renal Prostaglandin" rule:
- Diuretics need prostaglandins to work; NSAIDs (Option 4) block them.
- This represents a classic antagonistic interaction that every nurse must know.
- Rule out benign supplements and unrelated classes:
- Vitamin D (Option 1) and Penicillins (Option 3) do not interact with the Na+/K+/2Cl- pump or renal hemodynamics.
- While warfarin (Option 6) has many interactions, it is not traditionally contra-indicated or significantly altered by loop diuretics.
- Focus on metabolic stability: Always prioritize answers that involve blood sugar and potassium balance when dealing with diuretic interactions.
Take home points
- Loop diuretics antagonize the effects of antidiabetic medications by inducing hyperglycemia and reducing insulin sensitivity.
- The use of NSAIDs should be avoided or minimized because they inhibit renal prostaglandins and significantly reduce diuretic effectiveness.
- Concurrent use of corticosteroids and loop diuretics creates a synergistic effect on potassium excretion, increasing the risk of severe hypokalemia.
- Nurses must prioritize the monitoring of serum glucose and potassium levels whenever these interacting medications are prescribed together.
A nurse is caring for a client with renal disease who has an order for furosemide (Lasix). Which of the following actions by the nurse is most important?
Explanation
Furosemide is a potent loop diuretic that inhibits the Na+/K+/2Cl- symporter in the thick ascending limb. It is indicated for fluid overload and edema, but carries risks of nephrotoxicity and electrolyte depletion. Severe side effects include hypokalemia, ototoxicity, and metabolic alkalosis.
Rationale for correct answer
A. Patients with pre-existing renal disease are at a significantly higher risk for medication-induced kidney injury. The nurse must monitor urine output and creatinine levels to ensure the drug is not causing acute nephrotoxicity. Vigilant assessment allows for the early detection of declining glomerular filtration and potential dose adjustments.
Rationale for incorrect answers
B. Specific gravity measures the concentration of dissolved solutes in the urine. While it provides data on hydration status, it is not the most critical indicator of renal safety during furosemide therapy. Monitoring for acute injury through creatinine and output remains a higher clinical priority for this client.
C. Furosemide is a non-potassium-sparing diuretic that promotes the renal excretion of potassium ions. Eliminating potassium-rich foods would actually increase the risk of the patient developing severe hypokalemia. Patients typically require increased intake of this cation to maintain cardiac and muscular electrical stability.
D. Encouraging voiding every 4 hours is a general bladder health intervention. It does not address the systemic pharmacological risks associated with potent loop diuretics in a renal patient. Assessing the total volume of output is more scientifically relevant than the frequency of voiding episodes.
Test-taking strategy
- Identify the client's vulnerability: The question specifies "renal disease," which should immediately direct the focus toward monitoring for further organ damage or functional decline.
- Prioritize safety over routine care: While checking specific gravity (option 2) and voiding frequency (option 4) are nursing tasks, they are lower in the hierarchy of risk reduction compared to monitoring for drug-induced nephrotoxicity.
- Apply pharmacological knowledge: Recall that loop diuretics are potassium-wasting. This allows for the immediate elimination of option 3, as it suggests a counter-productive and dangerous nutritional intervention (eliminating potassium).
- Focus on the most important action: Look for the answer that provides the most direct assessment of the adverse effects specific to the medication and the patient's existing pathophysiology. Monitoring renal lab values like BUN and serum creatinine is the gold standard for assessing medication safety in renal failure.
Take home points
- Furosemide requires close monitoring of blood urea nitrogen and serum creatinine to prevent the progression of renal insufficiency.
- Loop diuretics typically cause hypokalemia, requiring patients to increase rather than decrease their dietary intake of potassium.
- Adequate urine output for an adult is defined as ≥ 30 mL/hr; levels below this may indicate drug-induced renal impairment.
- Serum electrolyte panels should be obtained frequently to screen for hyponatremia, hypocalcemia, and hypomagnesemia during active diuresis.
Exams on Drugs Used to Treat Heart Failure
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
Notes Highlighting is available once you sign in. Login Here.
Objectives
- Describe the pathophysiology of heart failure, including HFrEF and HFpEF classifications.
- Identify the clinical manifestations commonly associated with heart failure.
- Explain the goals of heart failure management, including symptom relief and mortality reduction.
- Differentiate between non-pharmacologic and pharmacologic strategies used in heart failure treatment.
- Explain the mechanism of action, clinical effects, and indications of ACE inhibitors in HFrEF.
- Describe the dual mechanism, benefits, and monitoring requirements of angiotensin receptor–neprilysin inhibitors (ARNIs).
- Explain how beta-blockers improve ventricular function and reduce mortality in chronic heart failure.
- Describe the inotropic and neurohormonal effects of digitalis glycosides, including digoxin.
- Explain the use, mechanism, and monitoring of phosphodiesterase inhibitors in acute decompensated heart failure.
- Identify the role, indications, adverse effects, and nursing considerations for miscellaneous heart failure agents such as ivabradine.
Introduction
HEART FAILURE
Heart failure is a complex clinical syndrome in which the heart is unable to pump sufficient blood to meet the metabolic, demands of the body or can do so only at elevated filling pressures. It is a progressive condition characterized by impaired ventricular filling or ejection of blood.
Heart failure is broadly classified into heart failure with reduced ejection fraction (HFrEF), defined by a left ventricular ejection fraction of less than 40 percent, and heart failure with preserved ejection fraction (HFpEF), in which the ejection fraction is 50 percent or greater but ventricular relaxation and filling are impaired.
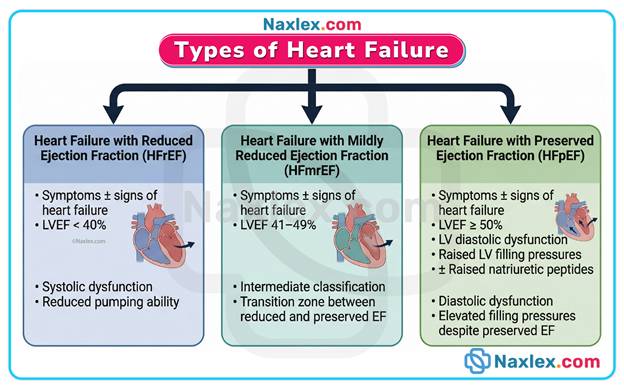
The syndrome results from structural or functional cardiac disorders such as ischemic heart disease, chronic hypertension, valvular heart disease, cardiomyopathy, and congenital heart defects.
Pathophysiology: Reduced cardiac output leads to decreased renal perfusion and activation of compensatory neurohormonal mechanisms, including the renin–angiotensin–aldosterone system (RAAS), the sympathetic nervous system, and antidiuretic hormone release.
Initially compensatory, chronic activation of these systems causes vasoconstriction, sodium and water retention, ventricular remodeling, myocardial fibrosis, and progressive deterioration of cardiac function.
Clinical manifestations include:
- Dyspnea
- Orthopnea
- Paroxysmal nocturnal dyspnea
- Peripheral edema
- Fatigue
- Weight gain
- Pulmonary crackles
- Jugular venous distension
- Decreased exercise tolerance
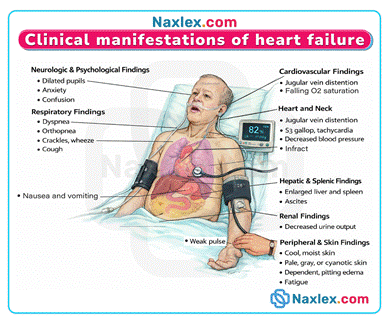
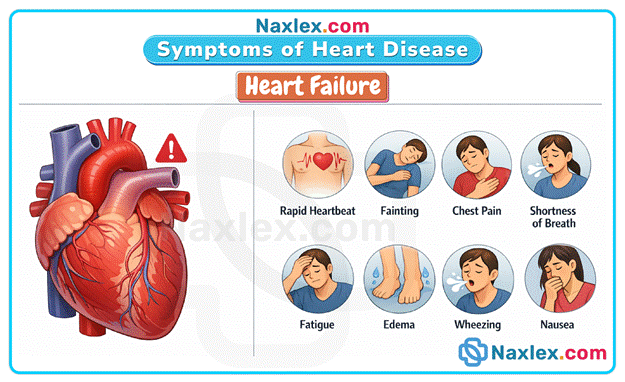
TREATMENT OF HEART FAILURE
The goals of heart failure management are to relieve symptoms, improve quality of life, reduce hospitalizations, slow disease progression, and reduce mortality.
Treatment involves a combination of non pharmacologic and pharmacologic strategies.
Non pharmacologic measures include:
- Dietary sodium restriction
- Fluid restriction in selected patients
- Weight management
- Smoking cessation
- Physical activity as tolerated
- Management of comorbid conditions such as hypertension, diabetes mellitus, and coronary artery disease
Pharmacologic therapy is the cornerstone of heart failure management and targets the underlying neurohormonal dysregulation, volume overload, and impaired myocardial contractility.
Evidence-based drug therapy has been shown to significantly improve survival in patients with HFrEF.
Most patients require combination therapy because no single drug adequately addresses all pathophysiologic mechanisms involved in heart failure.
Drug Therapy For Heart Failure
Heart failure is treated with a combination of vasodilator, diuretic, and inotropic therapy.
- Vasodilators reduce preload and afterload, thereby decreasing myocardial workload and improving cardiac output.
- Diuretics relieve symptoms of congestion by promoting sodium and water excretion.
- Inotropic agents improve myocardial contractility in patients with reduced cardiac output.
Modern heart failure therapy also includes agents that modulate neurohormonal activation and prevent adverse ventricular remodeling. Major drug classes used in the management of heart failure include:
- Angiotensin converting enzyme inhibitors (ACE inhibitors)
- Angiotensin receptor blocker–neprilysin inhibitors (ARNIs)
- Beta adrenergic blocking agents
- Digitalis glycosides
- Phosphodiesterase inhibitors
- Selected miscellaneous agents
These drugs are often used concurrently, with dosing individualized based on disease severity, renal function, blood pressure, and patient tolerance.
NURSING IMPLICATIONS FOR HEART FAILURE THERAPY
Nurses play a critical role in the management of patients with heart failure. Ongoing assessment includes monitoring:
- Vital signs
- Daily weight
- Intake and output
- Lung sounds
- Peripheral edema
- Signs of worsening heart failure
Blood pressure and heart rate must be monitored closely, particularly when initiating or titrating vasodilators and beta blockers. Laboratory monitoring includes serum electrolytes, renal function tests, and drug-specific levels such as serum digoxin concentration.
Patient education is essential and should emphasize:
- Medication adherence
- Recognition of early signs of fluid overload
- Dietary sodium restriction
- Importance of daily weight monitoring
Patients should be instructed to report:
- Rapid weight gain
- Increasing dyspnea
- Dizziness
- Palpitations promptly
Nurses must also assess for adverse drug reactions and potential drug interactions, especially in older adults who are often receiving multiple medications.
Angiotensin-Converting Enzyme (ACE) Inhibitors
Examples:
• captopril (Capoten)
• enalapril (Vasotec)
• lisinopril (Prinivil, Zestril)
• ramipril (Altace)
• perindopril (Coversyl)
• quinapril (Accupril)
• fosinopril (Monopril)
ACE inhibitors are cornerstone drugs in the management of chronic heart failure and are recommended for all patients with heart failure with reduced ejection fraction (HFrEF), unless contraindicated.
Mechanism of Action & Drug Effects:
ACE inhibitors block the angiotensin-converting enzyme, which is responsible for converting angiotensin I into angiotensin II, a potent vasoconstrictor.
In heart failure, the renin–angiotensin–aldosterone system (RAAS) is chronically activated, contributing to disease progression.
By inhibiting ACE, these drugs produce the following effects:
• Reduced angiotensin II levels – leads to arterial and venous vasodilation, decreasing systemic vascular resistance (afterload).
• Decreased aldosterone secretion – promotes sodium and water excretion, reducing preload and congestion.
• Reduced sympathetic nervous system activation – lowers heart rate and myocardial oxygen demand.
• Decreased vasopressin release – further limits water retention.
• Increased bradykinin levels – enhances vasodilation and nitric oxide release (also responsible for cough and angioedema).
• Prevention of ventricular remodeling – limits myocardial hypertrophy and fibrosis, slowing progression of heart failure.
Overall hemodynamic effects in heart failure:
• Decreased preload
• Decreased afterload
• Improved cardiac output
• Improved exercise tolerance
Clinical Significance:
ACE inhibitors significantly reduce morbidity and mortality in patients with heart failure.
They improve symptoms, reduce hospitalizations, slow disease progression, and prolong survival.
Their ability to prevent maladaptive cardiac remodeling makes them disease-modifying agents rather than purely symptomatic treatments.
As a result, ACE inhibitors are first-line therapy in chronic heart failure unless contraindicated.
Indications:
In the context of heart failure, ACE inhibitors are indicated for:
• Chronic heart failure with reduced ejection fraction (HFrEF) – first-line therapy in all symptomatic and asymptomatic patients.
• Asymptomatic left ventricular systolic dysfunction – prevents progression to symptomatic heart failure.
• Heart failure following myocardial infarction – reduces mortality and ventricular remodeling.
• Adjunct therapy in severe heart failure – used in combination with diuretics, beta-blockers, aldosterone antagonists, and other guideline-directed therapies.
Nursing relevance:
• ACE inhibitors are started at low doses and titrated upward based on tolerance.
• They are continued indefinitely unless adverse effects or contraindications develop.
Adverse Effects:
Common effects:
• Hypotension – especially after the first dose (“first-dose hypotension”), more pronounced in volume-depleted patients.
• Dizziness or lightheadedness
• Dry, persistent cough – due to accumulation of bradykinin.
• Fatigue
Serious or potentially life-threatening effects:
• Angioedema – rapid swelling of the face, lips, tongue, or airway; can be fatal.
• Hyperkalemia – due to reduced aldosterone-mediated potassium excretion.
• Acute kidney injury – particularly in patients with bilateral renal artery stenosis or severe renal hypoperfusion.
• Severe hypotension – especially in patients on high-dose diuretics.
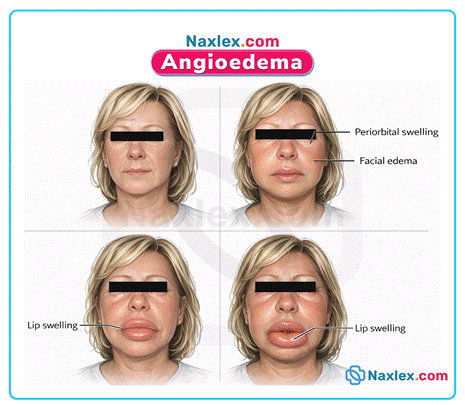
Nursing Tip: A persistent dry cough is common and benign, but angioedema is a medical emergency requiring immediate drug discontinuation and urgent care.
Contraindications / Precautions:
• History of angioedema related to ACE inhibitor therapy.
• Pregnancy – ACE inhibitors are teratogenic and can cause fetal renal failure and death.
• Bilateral renal artery stenosis – risk of acute renal failure.
• Severe hyperkalemia (potassium ≥5.5 mmol/L).
• Symptomatic hypotension.
Precautions:
• Renal impairment – start at low doses and monitor renal function closely.
• Elderly patients – increased risk of hypotension and renal effects.
• Volume depletion or hyponatremia – correct before initiation to reduce hypotension risk.
Drug Interactions:
• Potassium-sparing diuretics or potassium supplements – increased risk of hyperkalemia.
• Aldosterone antagonists (spironolactone, eplerenone) – additive potassium retention; requires close monitoring.
• NSAIDs – may reduce antihypertensive effect and increase risk of renal dysfunction.
• Lithium – ACE inhibitors can increase lithium levels, leading to toxicity.
• Other antihypertensives – additive hypotensive effects.
Nursing interventions:
• Review all medications and supplements, including over-the-counter NSAIDs and potassium products.
• Monitor potassium and renal function closely when combination therapy is necessary.
Nursing Insight:
• Administer the first dose at bedtime to reduce the risk of severe hypotension.
• Assess blood pressure before each dose, especially during initiation and titration.
• Educate patients to rise slowly from sitting or lying positions to prevent orthostatic hypotension.
• Instruct patients to report persistent cough, facial swelling, difficulty breathing, or dizziness.
• Emphasize adherence, as benefits are long-term and not always immediately noticeable.
• Reinforce that ACE inhibitors improve survival, not just symptoms, in heart failure.
Rationale:
Consistent use of ACE inhibitors counteracts harmful neurohormonal activation in heart failure, thereby improving long-term outcomes and reducing disease progression.
Monitoring & Evaluation:
• Blood pressure – baseline and ongoing monitoring to detect hypotension.
• Renal function – serum creatinine and estimated GFR at baseline, 1–2 weeks after initiation or dose changes, and periodically thereafter.
• Serum potassium – monitor for hyperkalemia, especially in patients on potassium-sparing agents.
• Clinical status – assess for reduced dyspnea, improved exercise tolerance, and decreased edema.
• Adverse effects – monitor for cough, angioedema, dizziness, and signs of renal dysfunction.
• Therapeutic effectiveness – evaluated by symptom improvement, reduced hospitalizations, and stabilization or improvement of heart failure severity.
Angiotensin Receptor Blocker–Neprilysin Inhibitor (ARNI)
Examples:
• sacubitril/valsartan (Entresto)
ARNI therapy represents a major advancement in the pharmacologic management of chronic heart failure and is specifically indicated for patients with HFrEF who remain symptomatic despite standard therapy.
Mechanism of Action & Drug Effects:
Angiotensin receptor blocker–neprilysin inhibitors combine two complementary mechanisms in a single agent. Sacubitril is a neprilysin inhibitor, while valsartan is an angiotensin II receptor blocker (ARB).
Neprilysin is an enzyme responsible for the breakdown of endogenous vasoactive peptides such as natriuretic peptides, bradykinin, and adrenomedullin. Inhibition of neprilysin leads to increased levels of these beneficial peptides, promoting natriuresis, diuresis, and vasodilation.
The ARB component blocks angiotensin II at the AT1 receptor, reducing vasoconstriction, aldosterone secretion, sodium retention, and sympathetic activation.
Combined effects include:
• Reduced preload and afterload
• Enhanced sodium and water excretion
• Suppression of maladaptive neurohormonal activation
• Reduced cardiac remodeling and fibrosis
• Improved cardiac efficiency and functional capacity
Clinical Significance:
In patients with chronic heart failure, ARNI therapy has been shown to significantly reduce cardiovascular mortality and heart failure–related hospitalizations compared with ACE inhibitor therapy alone. This dual-action approach targets both harmful and protective neurohormonal pathways, making ARNI therapy a preferred option in eligible patients.
Indications:
In the context of heart failure, angiotensin receptor blocker–neprilysin inhibitors are indicated for:
• Chronic heart failure with reduced ejection fraction (HFrEF) – as a replacement for ACE inhibitors or ARBs in stable patients.
• Symptomatic heart failure (NYHA class II–III) despite optimal conventional therapy.
• Patients tolerating ACE inhibitors or ARBs who require further risk reduction.
Nursing relevance:
• ARNI therapy should not be initiated concurrently with an ACE inhibitor.
• A washout period of at least 36 hours is required when switching from an ACE inhibitor to an ARNI.
Adverse Effects:
Common effects:
• Hypotension – due to potent vasodilatory effects.
• Dizziness or lightheadedness
• Fatigue
Serious or potentially life-threatening effects:
• Angioedema – risk increased in patients previously exposed to ACE inhibitors.
• Hyperkalemia – due to reduced aldosterone activity.
• Worsening renal function – particularly in patients with pre-existing renal impairment.
Nursing Tip: Facial swelling, tongue swelling, or difficulty breathing requires immediate discontinuation of therapy and emergency evaluation.
Contraindications / Precautions:
• History of angioedema related to ACE inhibitor or ARB therapy.
• Concomitant use with ACE inhibitors.
• Pregnancy – associated with fetal toxicity and death.
• Severe hepatic impairment.
Precautions:
• Renal impairment – initiate at lower doses and monitor renal function closely.
• Volume depletion – increases risk of symptomatic hypotension.
Drug Interactions:
• ACE inhibitors – increased risk of angioedema; contraindicated.
• Potassium supplements or potassium-sparing diuretics – increased risk of hyperkalemia.
• NSAIDs – increased risk of renal dysfunction.
• Other antihypertensive agents – additive blood pressure–lowering effects.
Nursing interventions:
• Verify appropriate washout period before initiation.
• Monitor renal function and serum potassium regularly.
Nursing Insight:
• Initiate therapy at the lowest recommended dose and titrate gradually.
• Educate patients to report dizziness, fainting, or facial swelling promptly.
• Reinforce adherence, as clinical benefits depend on consistent long-term use.
• Emphasize that ARNI therapy improves outcomes beyond symptom control alone.
Rationale:
By enhancing endogenous natriuretic peptide activity while suppressing angiotensin II effects, ARNI therapy provides balanced neurohormonal modulation and superior protection against heart failure progression.
Monitoring & Evaluation:
• Blood pressure – baseline and ongoing monitoring for symptomatic hypotension.
• Renal function – serum creatinine and estimated GFR at baseline and periodically thereafter.
• Serum potassium – monitor for hyperkalemia, especially with combination therapy.
• Clinical status – assess for improved exercise tolerance, reduced dyspnea, and decreased edema.
• Therapeutic effectiveness – measured by symptom improvement, reduced hospitalizations, and stabilization of heart failure severity.
Beta-Adrenergic Blocking Agents (Beta-Blockers)
Examples:
• carvedilol (Coreg)
• metoprolol succinate (Toprol XL)
• bisoprolol (Zebeta)
Beta-adrenergic blocking agents are a foundational component of guideline-directed medical therapy for chronic heart failure and are proven to reduce mortality in patients with HFrEF when used appropriately.
Mechanism of Action & Drug Effects:
Beta-blockers act by antagonizing beta-1 adrenergic receptors in the heart, counteracting chronic sympathetic nervous system activation that characterizes heart failure. Persistent adrenergic stimulation leads to tachycardia, increased myocardial oxygen demand, and progressive ventricular dysfunction.
By blocking beta-1 receptors, beta-blockers produce the following effects:
• Reduction in heart rate and myocardial contractility, decreasing oxygen demand.
• Suppression of harmful catecholamine effects on the myocardium.
• Improved ventricular filling time during diastole.
• Inhibition of pathologic cardiac remodeling and apoptosis.
• Improved left ventricular function over time.
Hemodynamic effects in chronic heart failure include:
• Reduced preload due to improved ventricular relaxation.
• Reduced afterload through decreased renin release and sympathetic tone.
• Gradual improvement in cardiac output with long-term therapy.
Clinical Significance:
Although beta-blockers may initially worsen symptoms due to negative inotropic effects, long-term use results in improved survival, reduced hospitalizations, and reversal of ventricular dysfunction. They are disease-modifying agents that directly oppose the neurohormonal mechanisms driving heart failure progression.
Indications:
In the context of heart failure, beta-adrenergic blocking agents are indicated for:
• Chronic heart failure with reduced ejection fraction (HFrEF) – stable patients without acute decompensation.
• Asymptomatic left ventricular systolic dysfunction.
• Heart failure following myocardial infarction.
• Adjunct therapy with ACE inhibitors or ARNI, diuretics, and aldosterone antagonists.
Nursing relevance:
• Beta-blockers must be initiated at very low doses and titrated slowly.
• Patients should be clinically stable and euvolemic before initiation.
Adverse Effects:
Common effects:
• Hypotension
• Bradycardia
• Fatigue
• Dizziness
Serious or clinically significant effects:
• Worsening heart failure symptoms during early therapy.
• Atrioventricular block in susceptible patients.
• Masking of hypoglycemia symptoms in diabetic patients.
Nursing Tip: Initial symptom worsening is expected; beta-blockers should not be abruptly discontinued unless severe decompensation occurs.
Contraindications / Precautions:
• Acute decompensated heart failure.
• Severe bradycardia or advanced heart block without a pacemaker.
• Symptomatic hypotension.
• Severe asthma or reactive airway disease (non-selective agents).
Image title: Beta blockers Broncho-constriction
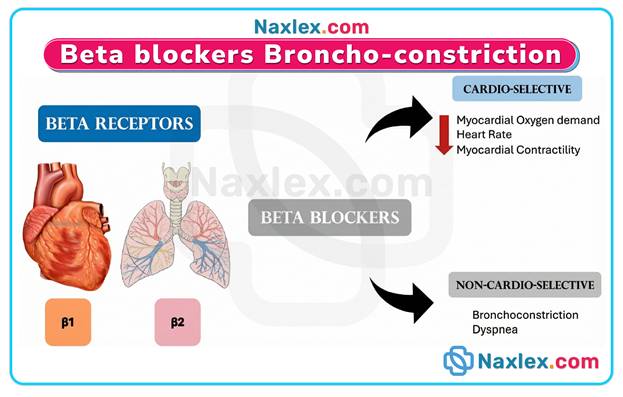
Precautions:
• Diabetes mellitus – may mask adrenergic symptoms of hypoglycemia.
• Peripheral vascular disease – may worsen symptoms.
Drug Interactions:
• Calcium channel blockers (verapamil, diltiazem) – increased risk of bradycardia and heart block.
• Other antihypertensive agents – additive hypotensive effects.
• Digoxin – increased risk of bradycardia.
Nursing interventions:
• Assess heart rate and blood pressure before each dose.
• Hold medication and notify provider if heart rate or blood pressure is below prescribed parameters.
Nursing Insight:
• Beta-blockers should never be stopped abruptly due to risk of rebound sympathetic activation.
• Educate patients that benefits are delayed and require consistent use.
• Reinforce adherence, even if symptoms do not immediately improve.
• Monitor for gradual improvement in exercise tolerance over weeks to months.
Rationale:
By suppressing chronic sympathetic overstimulation, beta-blockers reduce myocardial injury, improve ventricular performance, and slow progression of heart failure.
Monitoring & Evaluation:
• Heart rate and blood pressure – baseline and ongoing monitoring.
• Clinical status – assess for worsening dyspnea, edema, or weight gain during initiation.
• Functional capacity – evaluate exercise tolerance and fatigue levels.
• Therapeutic effectiveness – measured by improved symptoms, reduced hospitalizations, and enhanced survival.
Digitalis Glycosides (Digoxin)
Examples:
• digoxin (Lanoxin)
Digitalis glycosides are positive inotropic agents used in the management of chronic heart failure, particularly in patients with reduced ejection fraction who remain symptomatic despite standard therapy.
Mechanism of Action & Drug Effects:
Digitalis glycosides inhibit the sodium–potassium ATPase pump in cardiac myocytes.
This inhibition leads to an increase in intracellular sodium, which indirectly raises intracellular calcium via the sodium–calcium exchanger.
Elevated calcium enhances myocardial contractility.
Key pharmacologic effects include:
• Increased force of myocardial contraction, improving cardiac output.
• Reduced ventricular end-diastolic volume due to improved systolic performance.
• Enhanced renal perfusion secondary to increased cardiac output.
• Decreased sympathetic nervous system activity.
• Reduced activation of the renin–angiotensin–aldosterone system.
Additionally, digoxin exerts parasympathomimetic effects on the atrioventricular node, contributing to heart rate control in certain patients.
Clinical Significance:
In heart failure, digitalis glycosides improve symptoms such as fatigue and dyspnea by enhancing cardiac output.
They do not reduce mortality but decrease hospitalizations and improve quality of life when added to guideline-directed therapy.
Indications:
Digitalis glycosides are indicated for:
• Chronic heart failure with reduced ejection fraction (HFrEF).
• Patients who remain symptomatic despite ACE inhibitors or ARNI, beta-blockers, and diuretics.
• Heart failure patients with concomitant atrial fibrillation requiring ventricular rate control.
Nursing relevance:
• Digoxin is not first-line therapy but used as adjunctive treatment.
• Dosing must be individualized due to a narrow therapeutic index.
Adverse Effects:
Digoxin toxicity is dose-related and potentially life-threatening.
Common effects:
• Nausea and vomiting
• Anorexia
• Fatigue
• Headache
Cardiac effects:
• Bradycardia
• Atrioventricular block
• Ventricular dysrhythmias
Neurologic and visual effects:
• Confusion
• Visual disturbances (blurred vision, yellow or green halos)
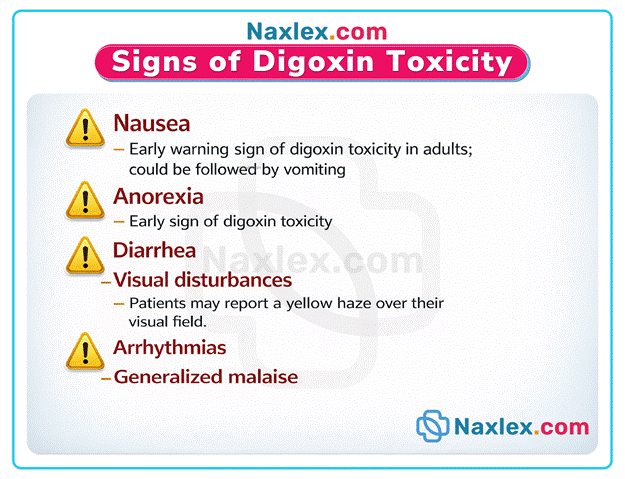
Nursing Tip: Early recognition of toxicity is essential due to the narrow therapeutic window.
Contraindications / Precautions:
• Ventricular fibrillation.
• Digoxin toxicity or hypersensitivity.
• Caution in renal impairment, as digoxin is primarily renally excreted.
• Electrolyte imbalances such as hypokalemia, hypomagnesemia, or hypercalcemia increase toxicity risk.
• Elderly patients are particularly susceptible to adverse effects.
Drug Interactions:
• Loop and thiazide diuretics – increased risk of hypokalemia and digoxin toxicity.
• Amiodarone, verapamil, quinidine – increase digoxin serum levels.
• ACE inhibitors – may alter potassium levels, affecting digoxin safety.
• Calcium supplements – may increase dysrhythmia risk.
Nursing Insight:
• Assess apical pulse for one full minute before administration; hold medication if heart rate is below prescribed parameters.
• Educate patients to recognize early signs of toxicity.
• Ensure consistent dosing time each day.
• Monitor renal function and electrolyte levels regularly.
• Reinforce adherence and discourage dose doubling if a dose is missed.
Rationale:
By improving myocardial contractility and reducing neurohormonal activation, digitalis glycosides provide symptomatic relief in chronic heart failure without increasing myocardial oxygen demand.
Monitoring & Evaluation:
• Serum digoxin levels – therapeutic range typically 0.5–0.9 ng/mL for heart failure.
• Electrolytes – potassium, magnesium, and calcium levels should be monitored closely.
• Renal function – monitor serum creatinine and estimated GFR.
• Heart rate and rhythm – assess for bradycardia or dysrhythmias.
• Clinical response – evaluate improvement in symptoms, exercise tolerance, and reduction in hospitalizations.
Phosphodiesterase Inhibitors (PDE Inhibitors)
Examples:
• milrinone (Primacor)
• inamrinone (Inocor)
These agents are short-term intravenous medications used primarily in acute decompensated heart failure when conventional therapy is insufficient.
Mechanism of Action & Drug Effects:
Phosphodiesterase inhibitors block the enzyme phosphodiesterase type 3 in cardiac and vascular smooth muscle. Inhibition of this enzyme prevents the breakdown of cyclic adenosine monophosphate (cAMP), leading to increased intracellular calcium availability in myocardial cells.
Key pharmacologic effects include:
• Positive inotropic effect – increases myocardial contractility, improving cardiac output.
• Positive lusitropic effect – enhances ventricular relaxation during diastole, improving ventricular filling.
• Peripheral vasodilation – decreases systemic vascular resistance, reducing afterload.
• Reduction in pulmonary vascular resistance – improves right ventricular function in patients with pulmonary congestion.
• Improved cardiac output without significant increase in myocardial oxygen demand.
Clinical Significance:
By increasing contractility and decreasing afterload, phosphodiesterase inhibitors rapidly improve hemodynamics in patients with severe heart failure. They are particularly useful in patients with low-output states who are unresponsive to diuretics and vasodilators.
Indications:
Phosphodiesterase inhibitors are indicated for:
• Acute decompensated heart failure with reduced ejection fraction.
• Short-term management of severe systolic dysfunction.
• Heart failure following cardiac surgery with low cardiac output syndrome.
• Patients requiring inotropic support while awaiting advanced therapies such as mechanical circulatory support or transplantation.
Nursing relevance:
• These drugs are not intended for long-term therapy due to increased mortality with chronic use.
• They are typically administered in intensive care or high-dependency settings.
Adverse Effects:
Phosphodiesterase inhibitors have a narrow therapeutic margin.
Common effects:
• Hypotension due to vasodilation
• Headache
• Nausea
Severe or life-threatening effects:
• Arrhythmias – ventricular dysrhythmias due to increased intracellular calcium.
• Thrombocytopenia – more common with inamrinone.
• Excessive vasodilation leading to hypotension and shock.
• Worsening myocardial ischemia in patients with coronary artery disease.
Nursing Tip: Continuous ECG monitoring is essential due to the risk of dysrhythmias.
Contraindications / Precautions:
• Severe aortic or pulmonary valve stenosis – increased contractility may worsen outflow obstruction.
• Hypovolemia – vasodilation may precipitate profound hypotension.
• History of serious ventricular arrhythmias.
• Caution in renal impairment – reduced clearance increases toxicity risk.
Nursing implication:
• Correct volume depletion before initiation.
• Dose adjustments are required in renal dysfunction.
Drug Interactions:
• Other inotropes – additive effects increase arrhythmia risk.
• Vasodilators – may potentiate hypotension.
• Diuretics – electrolyte disturbances (especially hypokalemia) increase dysrhythmia risk.
Nursing interventions:
• Monitor potassium and magnesium levels closely.
• Avoid rapid dose escalation.
Nursing Insight:
• Administer via continuous IV infusion using an infusion pump.
• Monitor blood pressure frequently due to risk of sudden hypotension.
• Assess urine output as an indirect indicator of improved cardiac output.
• Ensure continuous cardiac monitoring throughout therapy.
• Educate patients that therapy is temporary and hospital-based only.
Rationale:
These agents improve hemodynamics rapidly but carry significant risks, necessitating close monitoring in controlled settings.
Monitoring & Evaluation:
• Hemodynamic parameters – cardiac output, systemic vascular resistance, and pulmonary pressures when available.
• ECG monitoring – detect early ventricular dysrhythmias.
• Blood pressure – monitor for excessive vasodilation.
• Renal function – serum creatinine and urine output.
• Clinical response – improvement in dyspnea, perfusion, and end-organ function.
Miscellaneous Agents
Examples:
• ivabradine (Corlanor)
• omecamtiv mecarbil (Investigational cardiac myosin activator)
• vericiguat (BAY 1021189 – soluble guanylate cyclase stimulator)
These drugs represent adjunctive therapy for heart failure, particularly in patients who remain symptomatic despite standard therapy (ACE inhibitors, beta-blockers, diuretics, and mineralocorticoid receptor antagonists).
Mechanism of Action & Drug Effects:
Ivabradine: selectively inhibits the funny current (If) in the sinoatrial node, reducing heart rate without affecting myocardial contractility or blood pressure.
Omecamtiv mecarbil: increases cardiac myosin-actin cross-bridge formation, improving systolic contraction and stroke volume without increasing intracellular calcium.
Vericiguat: stimulates soluble guanylate cyclase, enhancing cyclic GMP production, leading to vasodilation and improved myocardial efficiency.
Key pharmacologic effects include:
• Heart rate reduction (ivabradine) – decreases myocardial oxygen demand and improves filling time.
• Improved cardiac output (omecamtiv mecarbil) – enhances systolic function in reduced ejection fraction heart failure.
• Vasodilation (vericiguat) – reduces afterload and myocardial workload.
• Symptom relief – decreases fatigue, dyspnea, and exercise intolerance.
• Adjunctive role – used in patients already on guideline-directed medical therapy (GDMT) with persistent symptoms.
Clinical Significance:
These agents provide targeted therapy for patients with HFrEF who cannot tolerate additional beta-blockade or remain symptomatic despite optimized therapy. Ivabradine is particularly useful in patients with elevated resting heart rate (>70 bpm) in sinus rhythm. Omecamtiv mecarbil and vericiguat are emerging therapies aimed at improving contractility and reducing heart failure hospitalizations.
Indications:
• Symptomatic HFrEF (EF ≤35%) despite optimal GDMT.
• Ivabradine – patients in sinus rhythm with resting heart rate ≥70 bpm.
• Omecamtiv mecarbil – investigational use in systolic dysfunction with low cardiac output.
• Vericiguat – chronic HFrEF patients recently hospitalized or requiring IV diuretics for decompensation.
• Adjunct therapy to standard care for symptom improvement and reduced hospitalizations.
Nursing relevance:
• Monitor heart rate closely with ivabradine; do not use in atrial fibrillation.
• Observe for signs of excessive hypotension with vericiguat.
• Educate patients on investigational drug status for agents like omecamtiv mecarbil.
Adverse Effects:
Common effects:
• Bradycardia (ivabradine)
• Visual disturbances (phosphenes – ivabradine)
• Fatigue, dizziness (all agents)
Severe or rare effects:
• Excessive bradycardia – may precipitate syncope (ivabradine)
• Hypotension – vericiguat, especially with concurrent vasodilators
• Arrhythmias – rare with omecamtiv mecarbil
• Headache and gastrointestinal upset
Nursing Tip: Regular monitoring of vital signs and ECG is essential during therapy.
Contraindications / Precautions:
• Acute decompensated heart failure – initiation during hemodynamic instability is unsafe.
• Heart rate <60 bpm (ivabradine) – contraindicated due to risk of severe bradycardia.
• Atrial fibrillation – ivabradine ineffective; omit therapy.
• Hypotension (SBP <100 mmHg) – caution with vericiguat.
• Pregnancy and lactation – use only if benefits outweigh risks.
Drug Interactions:
• CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin) – increase ivabradine levels, risk of bradycardia.
• Other heart rate–lowering agents – additive bradycardia risk.
• Vasodilators – additive hypotension with vericiguat.
Nursing interventions:
• Monitor heart rate and blood pressure before and during therapy.
• Assess for visual changes with ivabradine.
• Ensure patient adherence and educate on dosing schedule.
• Monitor for signs of worsening heart failure or hypotension.
• Report any syncope, dizziness, or palpitations immediately.
Nursing Insight:
• These agents are adjunctive therapy – not first-line monotherapy.
• Patient education is critical for safety and adherence.
• Monitor response via symptom relief, exercise tolerance, and hospitalization frequency.
• IV administration may be required for investigational agents in controlled settings.
• Encourage ongoing follow-up for dose titration and monitoring of adverse effects.
Rationale:
These agents target specific pathways in heart failure pathophysiology, complementing standard therapy to improve cardiac function, reduce symptoms, and prevent hospitalizations.
Monitoring & Evaluation:
• Vital signs – heart rate, blood pressure, rhythm.
• ECG – especially with ivabradine and omecamtiv mecarbil.
• Symptom assessment – fatigue, dyspnea, exercise tolerance.
• Hospitalization frequency – track effectiveness of adjunctive therapy.
• Laboratory monitoring – renal function and electrolytes as indicated.
Summary
HEART FAILURE OVERVIEW
- Complex clinical syndrome: heart cannot pump sufficient blood to meet metabolic demands.
- HFrEF: ejection fraction <40%; impaired systolic function.
- HFpEF: ejection fraction ≥50%; impaired ventricular relaxation/filling.
- Causes: ischemic heart disease, chronic hypertension, valvular disease, cardiomyopathy, congenital defects.
- Pathophysiology: decreased cardiac output → activates RAAS, sympathetic nervous system, ADH → vasoconstriction, fluid retention, remodeling, fibrosis.
- Clinical manifestations:
- Dyspnea, orthopnea, PND
- Peripheral edema
- Fatigue, weight gain
- Pulmonary crackles
- Jugular venous distension
- Decreased exercise tolerance
TREATMENT GOALS
- Relieve symptoms, improve quality of life, reduce hospitalizations.
- Slow disease progression and reduce mortality.
- Combination of non-pharmacologic and pharmacologic strategies.
NON-PHARMACOLOGIC STRATEGIES
- Sodium restriction, fluid restriction (select patients).
- Weight management, smoking cessation, physical activity as tolerated.
- Manage comorbidities: hypertension, diabetes, coronary artery disease.
PHARMACOLOGIC STRATEGIES
- Target neurohormonal dysregulation, volume overload, impaired contractility.
- Combination therapy usually required for optimal outcomes.
- Drug classes: ACE inhibitors, ARNIs, beta-blockers, digitalis glycosides, PDE inhibitors, miscellaneous agents.
ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS
- Examples: captopril, enalapril, lisinopril, ramipril.
- Mechanism: block conversion of angiotensin I → II → vasodilation, reduced aldosterone, decreased sympathetic activation, bradykinin increase.
- Effects: ↓ preload & afterload, ↑ cardiac output, prevent remodeling.
- Indications: HFrEF, asymptomatic LV dysfunction, post-MI, adjunct therapy.
- Adverse effects: hypotension, cough, angioedema, hyperkalemia, renal dysfunction.
- Nursing: start low dose, monitor BP, potassium, renal function; educate on cough, swelling.
ANGIOTENSIN RECEPTOR–NEPRILYSIN INHIBITORS (ARNIs)
- Example: sacubitril/valsartan.
- Dual mechanism: neprilysin inhibition ↑ natriuretic peptides; ARB blocks angiotensin II effects.
- Effects: vasodilation, natriuresis, reduced remodeling, improved cardiac efficiency.
- Indications: HFrEF, symptomatic despite ACE inhibitor/ARB.
- Nursing: 36-hour ACE inhibitor washout, monitor BP, renal function, potassium.
BETA-BLOCKERS
- Examples: carvedilol, metoprolol succinate, bisoprolol.
- Mechanism: block β1 receptors → ↓ heart rate, myocardial oxygen demand, suppress catecholamine injury.
- Effects: improved ventricular function, inhibit remodeling, gradual ↑ cardiac output.
- Indications: chronic HFrEF, post-MI, asymptomatic LV dysfunction.
- Nursing: start low dose, titrate slowly, monitor HR/BP; educate that benefits are delayed.
DIGITALIS GLYCOSIDES (DIGOXIN)
- Mechanism: inhibits Na+/K+ ATPase → ↑ intracellular calcium → ↑ contractility, ↓ sympathetic/RAAS activation.
- Effects: improved symptoms, reduced hospitalizations; no mortality benefit.
- Indications: HFrEF with persistent symptoms, atrial fibrillation for rate control.
- Adverse effects: nausea, bradycardia, AV block, visual disturbances.
- Nursing: monitor apical pulse, renal function, electrolytes; educate on toxicity signs.
PHOSPHODIESTERASE INHIBITORS
- Examples: milrinone, inamrinone (IV, short-term).
- Mechanism: ↑ cAMP → ↑ calcium → positive inotropy, lusitropy, vasodilation.
- Indications: acute decompensated HF, low-output states.
- Adverse effects: hypotension, arrhythmias, thrombocytopenia.
- Nursing: continuous ECG, monitor BP, electrolytes, renal function.
MISCELLANEOUS AGENTS
- Examples: ivabradine, omecamtiv mecarbil, vericiguat.
- Mechanisms:
- Ivabradine: inhibits If current → ↓ heart rate.
- Omecamtiv mecarbil: ↑ myosin-actin cross-bridges → ↑ systolic function.
- Vericiguat: stimulates soluble guanylate cyclase → vasodilation.
- Indications: symptomatic HFrEF despite optimal therapy.
- Adverse effects: bradycardia, visual disturbances, hypotension, arrhythmias.
- Nursing: monitor HR, BP, ECG; educate on dosing and symptom reporting.
NURSING CONSIDERATIONS
- Monitor: BP, HR, daily weight, intake/output, lung sounds, peripheral edema.
- Labs: electrolytes, renal function, drug-specific levels.
- Educate: adherence, symptom recognition, diet, daily weight, when to report:
- Rapid weight gain
- Increasing dyspnea
- Dizziness or palpitations
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Drugs Used to Treat Heart Failure
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


