Please set your exam date
Diabetes Mellitus
Study Questions
Practice Exercise 1
Which placental hormone is primarily responsible for increasing maternal insulin resistance during the second and third trimesters of pregnancy, contributing to the development of Gestational Diabetes Mellitus?
Explanation
Gestational Diabetes Mellitus is a glucose intolerance first recognized during pregnancy, typically emerging in the second or third trimester. It is driven by hormonal changes, particularly those that increase insulin resistance. The placenta secretes counter-regulatory hormones that antagonize insulin, including human placental lactogen, cortisol, and progesterone. These hormones peak between 24 and 28 weeks, impairing glucose uptake and increasing maternal blood glucose levels. The pancreas compensates by increasing insulin secretion, but if this fails, hyperglycemia ensues. Risk factors include obesity, polycystic ovarian syndrome, and a family history of diabetes. Fasting glucose ≥ 5.1 mmol/L or 1-hour post-OGTT ≥ 10.0 mmol/L confirms diagnosis.
Rationale for correct answer
3. Human placental lactogen (hPL) is secreted by the syncytiotrophoblast and rises progressively during pregnancy. It directly induces insulin resistance by antagonizing insulin receptors and promoting lipolysis, ensuring glucose availability for the fetus. Its peak action coincides with the onset of gestational diabetes, making it the primary hormone responsible.
Rationale for incorrect answers
1. Estrogen increases during pregnancy but does not significantly contribute to insulin resistance. Its primary roles include uterine growth and vascularization, not glucose metabolism. While it modulates insulin sensitivity indirectly, it lacks the potent anti-insulin effects of hPL.
2. Prolactin is secreted by the anterior pituitary and supports lactogenesis. It has minor effects on glucose metabolism, but it does not induce the level of insulin resistance required to cause gestational diabetes. Its concentration does not correlate with the timing of GDM onset.
4. Relaxin is involved in cervical ripening and pelvic ligament relaxation. It has no role in glucose regulation or insulin resistance. Its secretion pattern and physiological effects are unrelated to the metabolic changes seen in gestational diabetes.
Take home points
- Human placental lactogen is the key hormone driving insulin resistance in pregnancy.
- Gestational diabetes typically emerges in the second or third trimester due to hormonal shifts.
- Estrogen, prolactin, and relaxin have distinct roles unrelated to glucose metabolism.
- Diagnosis of GDM relies on specific glucose thresholds during oral glucose tolerance testing.
When discussing the pathophysiology of GDM, the nurse explains that the pancreas's inability to produce sufficient insulin to overcome increased insulin resistance is due to:
Explanation
Gestational Diabetes Mellitus arises when maternal insulin production fails to meet the increased metabolic demands of pregnancy. The placenta secretes anti-insulin hormones such as human placental lactogen, cortisol, and progesterone, which induce insulin resistance. Normally, pancreatic beta cells compensate by increasing insulin secretion. In GDM, this compensatory mechanism is impaired due to beta-cell dysfunction, leading to maternal hyperglycemia. Fasting plasma glucose ≥ 5.1 mmol/L, 1-hour OGTT ≥ 10.0 mmol/L, or 2-hour ≥ 8.5 mmol/L confirms diagnosis. Risk factors include obesity, prior GDM, and family history of type 2 diabetes.
Rationale for correct answer
3. Pancreatic beta-cell dysfunction is the primary defect in GDM. Beta cells fail to augment insulin secretion in response to rising insulin resistance. This dysfunction is due to genetic predisposition, chronic inflammation, and lipotoxicity. The question stem directly refers to the pancreas's inability to produce sufficient insulin, confirming beta-cell failure.
Rationale for incorrect answers
1. Hypertrophy of beta cells would imply increased insulin production. In GDM, the issue is not cell enlargement but functional impairment. Beta cells may appear morphologically normal or even hypertrophic, but their secretory capacity is inadequate.
2. Alpha-cell function relates to glucagon secretion, not insulin production. Alpha-cell impairment would affect counter-regulatory responses to hypoglycemia, not the hyperglycemia seen in GDM. The question focuses on insulin insufficiency, making alpha-cell dysfunction irrelevant.
4. Excessive glucagon secretion contributes to hyperglycemia but is not the primary defect in GDM. Glucagon increases hepatic glucose output, but the central issue is insulin resistance and inadequate beta-cell compensation. Glucagon excess is secondary and not causative.
Take home points
- Beta-cell dysfunction is the core defect in gestational diabetes.
- Insulin resistance in pregnancy is driven by placental hormones.
- Alpha cells regulate glucagon, not insulin.
- Glucagon excess may worsen hyperglycemia but is not the initiating factor.
What is the role of human placental lactogen in GDM pathophysiology?
Explanation
Gestational Diabetes Mellitus develops due to a mismatch between rising insulin resistance and inadequate beta-cell compensation during pregnancy. The placenta secretes counter-regulatory hormones that antagonize insulin, including human placental lactogen, cortisol, and progesterone. Human placental lactogen increases progressively and peaks in the third trimester, promoting lipolysis and impairing glucose uptake. This ensures maternal glucose availability for fetal growth. If pancreatic insulin secretion fails to match this resistance, maternal hyperglycemia results. Diagnosis is confirmed by OGTT thresholds: fasting ≥ 5.1 mmol/L, 1-hour ≥ 10.0 mmol/L, 2-hour ≥ 8.5 mmol/L.
Rationale for correct answer
2. Human placental lactogen enhances lipolysis and induces insulin resistance by antagonizing insulin receptors. This shifts maternal metabolism toward fat utilization while sparing glucose for fetal use. Its action is central to the pathophysiology of GDM, where insulin resistance exceeds pancreatic compensatory capacity.
Rationale for incorrect answers
1. Human placental lactogen does not increase insulin production. It impairs insulin action, requiring the pancreas to compensate. The hormone’s role is antagonistic to insulin, not stimulatory. Beta-cell compensation is a separate physiological response, not driven by hPL.
3. Human placental lactogen increases maternal glucose levels by reducing insulin sensitivity. It promotes glucose availability for the fetus, not reduction. Its metabolic effects oppose insulin, leading to elevated maternal glucose if insulin secretion is insufficient.
4. Human placental lactogen does not promote fetal hypoglycemia. It ensures fetal glucose supply by increasing maternal glucose levels. Fetal hypoglycemia may occur postpartum due to persistent fetal hyperinsulinemia, but this is unrelated to hPL’s direct action.
Take home points
- Human placental lactogen induces insulin resistance and lipolysis during pregnancy.
- GDM results from inadequate beta-cell compensation for rising insulin resistance.
- hPL increases maternal glucose, ensuring fetal nutrient supply.
- Fetal hypoglycemia is not caused by hPL but may follow maternal hyperglycemia.
When discussing the pathophysiology of Gestational Diabetes Mellitus, which of the following statements are accurate regarding the role of pregnancy hormones? Select all that apply
Explanation
Gestational Diabetes Mellitus is a pregnancy-induced glucose intolerance caused by rising insulin resistance and inadequate beta-cell compensation. Placental hormones such as human placental lactogen, cortisol, and progesterone exert anti-insulin effects, impairing maternal glucose uptake. This ensures a continuous glucose supply to the fetus. The maternal pancreas must increase insulin secretion to maintain euglycemia. If this fails, hyperglycemia develops. Diagnosis is confirmed by OGTT thresholds: fasting ≥ 5.1 mmol/L, 1-hour ≥ 10.0 mmol/L, 2-hour ≥ 8.5 mmol/L. Risk factors include obesity, prior GDM, and family history of diabetes.
Rationale for correct answers
2. Cortisol exhibits anti-insulin effects by promoting gluconeogenesis and impairing peripheral glucose uptake. It increases maternal blood glucose levels, contributing to insulin resistance during pregnancy. Its concentration rises progressively, peaking in the third trimester.
3. Human placental lactogen increases maternal insulin resistance by antagonizing insulin receptors and stimulating lipolysis. This spares glucose for fetal use and shifts maternal metabolism toward fat utilization. It is the most potent diabetogenic hormone in pregnancy.
5. These hormones ensure continuous glucose supply to the fetus by impairing maternal insulin action. This metabolic shift prioritizes fetal growth and development. Glucose crosses the placenta via facilitated diffusion, driven by maternal hyperglycemia.
Rationale for incorrect answers
1. Progesterone does not increase insulin sensitivity. It contributes to insulin resistance by interfering with insulin receptor signaling. Its role is synergistic with other placental hormones in reducing maternal glucose uptake.
4. Estrogen does not directly stimulate insulin production. It modulates vascular tone and supports uterine growth, but its influence on glucose metabolism is indirect. It may affect insulin sensitivity but does not enhance beta-cell secretion.
Take home points
- Cortisol and hPL are key drivers of insulin resistance in pregnancy.
- Progesterone contributes to insulin resistance, not sensitivity.
- Estrogen does not directly stimulate insulin production.
- Placental hormones ensure fetal glucose supply by impairing maternal insulin action.
Which of the following are key pathophysiological differences between preexisting diabetes mellitus and gestational diabetes mellitus in pregnancy? Select all that apply
Explanation
Gestational Diabetes Mellitus vs Preexisting Diabetes Mellitus represent distinct metabolic disorders with different onset, pathophysiology, and clinical implications. GDM arises from placental hormone-induced insulin resistance during pregnancy, typically after 20 weeks, and resolves postpartum. Preexisting diabetes, including type 1 and type 2, involves chronic hyperglycemia predating conception. Type 1 is due to autoimmune beta-cell destruction, while type 2 involves insulin resistance and relative insulin deficiency. Preexisting diabetes increases risk of congenital anomalies, especially if glycemic control is poor during organogenesis (weeks 5 to 8). GDM does not cause congenital defects but increases risk of macrosomia and neonatal hypoglycemia.
Rationale for correct answers
1. Preexisting diabetes involves chronic hyperglycemia that begins before conception. This includes both type 1 and type 2 diabetes. The presence of elevated glucose during embryogenesis increases risk of congenital malformations, especially cardiac and neural tube defects.
2. Gestational diabetes typically resolves postpartum because the diabetogenic placental hormones (human placental lactogen, cortisol, progesterone) decline after delivery. Insulin resistance diminishes, and glucose tolerance often normalizes unless underlying type 2 diabetes is unmasked.
4. Gestational diabetes arises from de novo insulin resistance in the second trimester due to rising levels of human placental lactogen, cortisol, and progesterone. This resistance peaks between 24 and 28 weeks, impairing maternal glucose uptake and increasing fetal glucose exposure.
Rationale for incorrect answers
3. Preexisting type 1 diabetes is not characterized by insulin resistance only. It is defined by absolute insulin deficiency due to autoimmune beta-cell destruction. Insulin resistance may occur later due to exogenous insulin use or obesity, but it is not the primary defect.
5. Preexisting diabetes carries a significant risk of congenital anomalies if glycemic control is poor during the first trimester. Hyperglycemia during organogenesis increases risk of cardiac defects, neural tube defects, and caudal regression syndrome. Tight glucose control before conception reduces this risk.
Take home points
- GDM arises from placental hormone-induced insulin resistance after 20 weeks.
- Preexisting diabetes involves chronic hyperglycemia and may cause congenital anomalies.
- Type 1 diabetes is defined by insulin deficiency, not resistance.
- GDM typically resolves postpartum unless underlying diabetes is present.
Practice Exercise 2
Which of the following best describes the key difference between preexisting type 1 diabetes mellitus and gestational diabetes mellitus in pregnancy?
Explanation
Diabetes in pregnancy involves distinct pathophysiologic mechanisms depending on whether the condition is preexisting or gestational. Type 1 diabetes is caused by autoimmune destruction of pancreatic β-cells, leading to absolute insulin deficiency. In contrast, gestational diabetes arises due to transient insulin resistance driven by placental hormones such as human placental lactogen, cortisol, and progesterone. This resistance peaks in the second and third trimesters. Type 1 diabetes is present before conception and carries risks of vascular complications such as retinopathy and nephropathy. Gestational diabetes typically resolves postpartum but increases future risk of type 2 diabetes. Fasting glucose ≥ 5.1 mmol/L or 1-hour post-load ≥ 10.0 mmol/L confirms gestational diabetes.
Rationale for correct answer
1. Preexisting type 1 diabetes is characterized by autoimmune β-cell destruction, resulting in complete lack of endogenous insulin. Gestational diabetes, however, is due to placental hormone-induced insulin resistance that develops during pregnancy and resolves postpartum. The question stem contrasts the underlying pathophysiology, making this the scientifically accurate distinction. Insulin deficiency and insulin resistance are the core mechanisms.
Rationale for incorrect answers
2. Type 1 diabetes always requires exogenous insulin therapy due to complete β-cell failure. Gestational diabetes may be managed with diet, exercise, or insulin depending on severity. The statement reverses the treatment reality. Insulin therapy is mandatory in type 1, not optional.
3. Type 1 diabetes is lifelong and does not resolve postpartum. Gestational diabetes typically resolves after delivery but increases risk for future type 2 diabetes. The statement incorrectly suggests type 1 is transient. Postpartum persistence applies to type 1, not gestational.
4. Vascular complications such as retinopathy, nephropathy, and cardiovascular disease are associated with long-standing type 1 diabetes. Gestational diabetes does not cause vascular damage unless poorly controlled or progresses to chronic diabetes. The statement misattributes vascular complications to gestational diabetes.
Take home points
- Type 1 diabetes involves autoimmune β-cell destruction and absolute insulin deficiency.
- Gestational diabetes results from placental hormone-induced insulin resistance.
- Type 1 diabetes requires lifelong insulin therapy; gestational diabetes may not.
- Vascular complications are common in type 1, not gestational diabetes.
A nurse is assessing a pregnant woman for risk factors of gestational diabetes. Which of the following is a non-modifiable risk factor?
Explanation
Gestational diabetes mellitus is a glucose intolerance first recognized during pregnancy. It results from placental hormones, insulin resistance, and beta-cell dysfunction. Risk increases with maternal age, family history, and ethnic predisposition. Symptoms are often absent but may include polyuria, polydipsia, and fatigue. Diagnosis is confirmed by a 2-hour oral glucose tolerance test ≥ 153 mg/dL. Early screening is essential in high-risk populations.
Rationale for correct answer
3. A family history of type 2 diabetes reflects a genetic predisposition to insulin resistance and pancreatic beta-cell dysfunction. This risk factor is non-modifiable because it is inherited and cannot be altered by lifestyle changes. The question stem asks for a risk factor that cannot be changed, making this the correct answer.
Rationale for incorrect answers
1. Obesity is a modifiable risk factor. Although it significantly increases insulin resistance and the risk of gestational diabetes, weight reduction before pregnancy can reduce this risk. Lifestyle interventions such as diet and exercise can alter this parameter.
2. A sedentary lifestyle contributes to insulin resistance and impaired glucose metabolism. However, it is a modifiable factor. Increasing physical activity improves insulin sensitivity and lowers the risk of gestational diabetes. Behavioral changes can directly impact this risk.
4. Excessive gestational weight gain is a modifiable factor. It can be controlled through nutritional counseling and regular monitoring during prenatal visits. Excess weight gain increases insulin resistance but is influenced by maternal behavior and clinical guidance.
Take home points
- Gestational diabetes risk increases with genetic predisposition and insulin resistance.
- Family history of type 2 diabetes is a non-modifiable risk factor.
- Obesity and sedentary lifestyle are modifiable contributors to gestational diabetes.
- Excessive gestational weight gain can be prevented with proper prenatal care.
A nurse is reviewing the risk factors for Gestational Diabetes Mellitus. Which maternal age is generally considered a risk factor?
Explanation
Gestational diabetes mellitus is a glucose intolerance first recognized during pregnancy. It results from placental hormones, insulin resistance, beta-cell dysfunction, and maternal metabolic stress. Risk increases with maternal age ≥ 35 years, especially in women with increased adiposity or family history of diabetes. Diagnosis is confirmed by a 2-hour oral glucose tolerance test ≥ 153 mg/dL. Screening is recommended between 24 and 28 weeks gestation.
Rationale for correct answer
3. Maternal age ≥ 35 years is a recognized independent risk factor for gestational diabetes due to progressive decline in insulin sensitivity and increased beta-cell workload. The question asks for the age generally considered a risk threshold, and ≥ 35 years is the standard cutoff used in clinical screening guidelines.
Rationale for incorrect answers
1. Age < 20 years is not associated with increased risk of gestational diabetes. Younger women typically have higher insulin sensitivity and lower metabolic stress. Although rare exceptions exist, this age group is not prioritized for early screening unless other risk factors are present.
2. Age < 35 years includes women under the threshold for increased risk. While some women in this group may develop gestational diabetes due to other factors, age alone is not sufficient to classify them as high risk. Clinical guidelines do not use this age as a screening trigger.
4. Age ≥ 40 years is a high-risk category, but it is not the general threshold used to define risk. While older maternal age increases insulin resistance and risk of glucose intolerance, the question asks for the age generally considered a risk factor, which is ≥ 35 years. This choice is too narrow and excludes the broader population at risk.
Take home points
- Gestational diabetes risk increases with maternal age ≥ 35 years.
- Age-related insulin resistance contributes to beta-cell dysfunction in pregnancy.
- Screening guidelines use ≥ 35 years as the threshold for risk-based testing.
- Age ≥ 40 years is high risk but not the general cutoff for screening.
Which of the following are risk factors for gestational diabetes? Select all that apply
Explanation
Gestational diabetes mellitus is a glucose intolerance first recognized during pregnancy. It arises from placental hormone antagonism, progressive insulin resistance, beta-cell dysfunction, and maternal metabolic stress. Risk factors include advanced maternal age ≥ 35 years, ethnic predisposition, and family history of type 2 diabetes. Diagnosis is confirmed by a 2-hour oral glucose tolerance test ≥ 153 mg/dL. Screening occurs between 24 and 28 weeks gestation.
Rationale for correct answers
1. Advanced maternal age increases insulin resistance and reduces beta-cell compensation. Women ≥ 35 years are at elevated risk due to age-related metabolic decline. This is a recognized screening criterion.
2. Family history of type 2 diabetes reflects genetic susceptibility to impaired glucose regulation. Inherited defects in insulin signaling and beta-cell function predispose to gestational diabetes.
4. Hispanic ethnicity is associated with higher prevalence of insulin resistance and gestational diabetes. Ethnic-specific genetic and environmental factors contribute to increased risk.
Rationale for incorrect answers
3. Low pre-pregnancy BMI is not a risk factor. Women with BMI < 18.5 typically have higher insulin sensitivity and lower adipose-derived inflammatory markers. They are less likely to develop gestational diabetes unless other risk factors are present.
5. Regular physical activity improves glucose metabolism and enhances insulin sensitivity. It is a protective factor, not a risk. Exercise reduces systemic inflammation and lowers gestational diabetes incidence.
Take home points
- Gestational diabetes risk increases with maternal age ≥ 35 years.
- Family history of type 2 diabetes is a strong genetic risk factor.
- Hispanic ethnicity is associated with higher gestational diabetes prevalence.
- Low BMI and regular exercise reduce risk through improved insulin sensitivity.
Which of the following risk factors are associated with an increased likelihood of developing Gestational Diabetes Mellitus? Select all that apply
Explanation
Gestational diabetes mellitus is a glucose intolerance first recognized during pregnancy. It results from placental hormone antagonism, progressive insulin resistance, beta-cell dysfunction, and maternal metabolic stress. Risk increases with obesity, family history of type 2 diabetes, and previous macrosomia. Diagnosis is confirmed by a 2-hour oral glucose tolerance test ≥ 153 mg/dL. Screening occurs between 24 and 28 weeks gestation.
Rationale for correct answers
1. Pre-pregnancy BMI ≥ 30 kg/m² indicates obesity, which increases insulin resistance and inflammatory cytokine activity. Adipose tissue impairs glucose uptake and promotes beta-cell stress, elevating gestational diabetes risk.
2. A previous large-for-gestational-age infant (> 4,000 g) suggests prior glucose intolerance or undiagnosed gestational diabetes. Fetal macrosomia reflects maternal hyperglycemia, increasing recurrence risk in subsequent pregnancies.
4. A first-degree relative with type 2 diabetes indicates genetic predisposition to impaired insulin signaling and beta-cell dysfunction. This familial link is a strong predictor of gestational diabetes development.
Rationale for incorrect answers
3. Maternal age < 20 years is not associated with increased gestational diabetes risk. Younger women typically have higher insulin sensitivity and lower metabolic burden. Unless other risk factors are present, this group is not prioritized for early screening.
5. History of hyperthyroidism does not directly increase gestational diabetes risk. While thyroid dysfunction can affect metabolic rate, hyperthyroidism is not linked to insulin resistance or glucose intolerance. It may complicate pregnancy but is not a recognized risk factor for gestational diabetes.
Take home points
- Obesity (BMI ≥ 30 kg/m²) increases insulin resistance and gestational diabetes risk.
- Prior macrosomia reflects maternal hyperglycemia and predicts recurrence.
- First-degree relatives with type 2 diabetes signal genetic susceptibility.
- Young maternal age and hyperthyroidism are not direct risk factors.
Practice Exercise 3
What is the purpose of the 50-gram, 1-hour glucose challenge test in GDM screening?
Explanation
Gestational diabetes mellitus (GDM) is a form of glucose intolerance that develops during pregnancy due to increased insulin resistance from placental hormones. Hyperglycemia, insulin resistance, placental hormones, and screening protocols are central to its pathophysiology. GDM typically emerges in the second trimester and is associated with macrosomia, shoulder dystocia, and neonatal hypoglycemia. Screening is essential because most women are asymptomatic. The 50-gram, 1-hour glucose challenge test is a non-fasting screening tool used between 24 and 28 weeks gestation. A plasma glucose level ≥140 mg/dL at 1 hour indicates the need for a confirmatory 3-hour oral glucose tolerance test.
Rationale for correct answer
2. The 50-gram, 1-hour glucose challenge test is a screening tool, not diagnostic. It identifies women who may have impaired glucose tolerance and require further testing. The test is performed without fasting, and a result ≥140 mg/dL prompts a 100-gram, 3-hour oral glucose tolerance test for diagnosis.
Rationale for incorrect answers
1. The glucose challenge test does not confirm GDM. It is a screening method. Diagnosis requires a 3-hour oral glucose tolerance test using 100 grams of glucose, with specific thresholds at fasting, 1 hour, 2 hours, and 3 hours. Confirmation needs at least 2 abnormal values.
3. The 50-gram glucose challenge test is not a fasting test. It is performed regardless of the last meal. Fasting glucose is measured separately and is not part of this screening. The test evaluates post-load glucose handling, not baseline glucose levels.
4. The test does not assess fetal growth. Fetal macrosomia is a consequence of poorly controlled GDM, but growth is monitored via ultrasound, not glucose challenge testing. The test evaluates maternal glucose metabolism, not fetal parameters.
Take home points
- The 50-gram, 1-hour glucose challenge test is a screening tool for GDM.
- A result ≥140 mg/dL requires a 3-hour oral glucose tolerance test.
- GDM is caused by placental hormone-induced insulin resistance.
- Fetal growth assessment is done via ultrasound, not glucose testing.
A primigravida client is being screened for Gestational Diabetes Mellitus at 26 weeks of gestation using the two-step approach. Her 1-hour 50-gram glucose challenge test result is 155 mg/dL. What is the most appropriate next step in her care?
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically in the second or third trimester. It results from placental hormones inducing insulin resistance, leading to hyperglycemia. Risk factors include obesity, age over 35 years, and family history of diabetes. GDM increases risk of macrosomia, shoulder dystocia, and neonatal hypoglycemia. Screening is done between 24 and 28 weeks using the two-step approach: first, a 50-gram glucose challenge test; if ≥140 mg/dL, proceed to a 100-gram, 3-hour oral glucose tolerance test. Diagnosis requires at least 2 abnormal values: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, 3-hour ≥140 mg/dL.
Rationale for correct answer
3. A 1-hour glucose challenge result of 155 mg/dL exceeds the screening threshold of 140 mg/dL. This indicates impaired glucose tolerance and necessitates a diagnostic 100-gram, 3-hour oral glucose tolerance test. The two-step protocol mandates confirmatory testing before any diagnosis or treatment decisions.
Rationale for incorrect answers
1. A value of 155 mg/dL is not normal. The cutoff for normal in the 50-gram challenge is <140 mg/dL. Reassurance without further testing risks missing subclinical hyperglycemia, which can lead to fetal complications if untreated.
2. Insulin therapy is not initiated based on screening results alone. Treatment begins only after confirmed diagnosis via the 3-hour oral glucose tolerance test. Premature insulin use may cause iatrogenic hypoglycemia and lacks diagnostic justification.
4. Dietary modifications are part of GDM management but not initiated at the screening stage. Without diagnostic confirmation, advising carbohydrate restriction is premature and may lead to nutritional imbalance in pregnancy.
Take home points
- A 50-gram glucose challenge result ≥140 mg/dL requires a 3-hour OGTT.
- GDM diagnosis needs 2 abnormal values from the 100-gram OGTT.
- Insulin is started only after confirmed diagnosis.
- Dietary changes are part of management, not screening.
When should high-risk women be screened for gestational diabetes?
Explanation
Gestational diabetes mellitus (GDM) is a pregnancy-induced glucose intolerance caused by placental hormones that antagonize insulin, leading to insulin resistance and maternal hyperglycemia. High-risk women include those with obesity, prior GDM, family history of type 2 diabetes, or polycystic ovary syndrome. Early screening is essential to prevent macrosomia, preeclampsia, and neonatal hypoglycemia. The two-step screening approach uses a 50-gram glucose challenge test followed by a 100-gram oral glucose tolerance test if needed. In high-risk women, screening should occur in the first trimester, typically before 13 weeks gestation, using fasting glucose or HbA1c.
Rationale for correct answer
2. High-risk women should be screened in the first trimester due to elevated baseline risk of glucose intolerance. Early detection allows for timely intervention and reduces complications. Fasting plasma glucose ≥92 mg/dL, HbA1c ≥5.7%, or random glucose ≥200 mg/dL may indicate early GDM or overt diabetes.
Rationale for incorrect answers
1. Screening at delivery is clinically irrelevant. By this time, undiagnosed GDM may have already caused fetal complications such as macrosomia or shoulder dystocia. Screening must occur early to allow for glycemic control during pregnancy.
3. Screening at 32 weeks is too late. GDM typically develops between 24 and 28 weeks due to rising placental hormones. Delayed screening increases risk of uncontrolled hyperglycemia and adverse outcomes. Early identification is critical in high-risk populations.
4. Postpartum screening is used to assess resolution of GDM or detect persistent type 2 diabetes. It is not appropriate for initial screening. During pregnancy, undiagnosed GDM can lead to neonatal hypoglycemia and maternal complications, making early screening essential.
Take home points
- High-risk women should be screened for GDM in the first trimester.
- Early screening uses fasting glucose, HbA1c, or random glucose.
- Screening at delivery or postpartum is not appropriate for diagnosis.
- GDM screening timing affects maternal and fetal outcomes.
Which test is used to screen for persistent glucose intolerance postpartum in women with GDM?
Explanation
Postpartum glucose intolerance screening is essential in women with prior gestational diabetes mellitus (GDM) due to increased risk of developing type 2 diabetes mellitus. Beta-cell dysfunction, insulin resistance, glucose intolerance, and long-term metabolic risk are central concerns. GDM resolves after delivery in most cases, but up to 50% of affected women develop type 2 diabetes within 5 to 10 years. The recommended screening method is the 75-gram, 2-hour oral glucose tolerance test performed at 6 to 12 weeks postpartum. This test evaluates both fasting and post-load glucose handling, detecting impaired fasting glucose and impaired glucose tolerance.
Rationale for correct answer
2. The 75-gram, 2-hour oral glucose tolerance test is the gold standard for postpartum screening. It assesses fasting and postprandial glucose levels, identifying both impaired fasting glucose and impaired glucose tolerance. It is performed at 6 to 12 weeks postpartum and is more sensitive than fasting glucose or HbA1c alone.
Rationale for incorrect answers
1. The 50-gram, 1-hour glucose challenge test is used for screening during pregnancy, not postpartum. It lacks diagnostic precision and does not provide fasting or 2-hour values. It cannot detect persistent glucose intolerance after delivery.
3. Fasting plasma glucose alone may miss cases of impaired glucose tolerance. While useful, it lacks post-load assessment, which is critical for identifying subtle abnormalities in glucose metabolism. It has lower sensitivity compared to the 2-hour OGTT.
4. Hemoglobin A1c reflects average glucose over 2 to 3 months but is less sensitive in the postpartum period due to rapid changes in glucose metabolism. It may miss early glucose dysregulation and is not recommended as a sole screening tool.
Take home points
- The 75-gram, 2-hour OGTT is the preferred postpartum screening test for GDM.
- Fasting glucose and HbA1c alone are less sensitive.
- The 50-gram test is used only during pregnancy.
- Postpartum screening should occur at 6 to 12 weeks.
Which of the following are diagnostic thresholds for the 100-gram, 3-hour OGTT in GDM? Select all that apply
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically in the second or third trimester. It results from placental hormones such as human placental lactogen, cortisol, and progesterone causing insulin resistance, leading to maternal hyperglycemia. Diagnosis is confirmed using the 100-gram, 3-hour oral glucose tolerance test (OGTT) after an abnormal 50-gram screening. The test is performed after an overnight fast and includes measurements at fasting, 1 hour, 2 hours, and 3 hours. Diagnostic thresholds are: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, and 3-hour ≥140 mg/dL. At least 2 values must be abnormal for diagnosis.
Rationale for correct answers
1. Fasting glucose ≥95 mg/dL is a diagnostic threshold for GDM in the 100-gram OGTT. This value reflects baseline glycemic control and is sensitive to early insulin resistance. It is measured after an 8-hour fast and is the first value assessed.
3. The 2-hour threshold of ≥155 mg/dL is part of the diagnostic criteria. It reflects post-load glucose clearance and is critical for identifying delayed insulin response. This value captures intermediate glucose handling after the peak.
4. The 3-hour threshold of ≥140 mg/dL is the final diagnostic point. It assesses sustained hyperglycemia and late-phase insulin activity. Persistence of elevated glucose at 3 hours indicates impaired glucose tolerance.
Rationale for incorrect answers
2. The correct 1-hour threshold is ≥180 mg/dL, not ≥170 mg/dL. A value of 170 mg/dL is below the diagnostic cutoff and may miss cases of early postprandial hyperglycemia. Using a lower threshold reduces specificity and alters diagnostic accuracy.
5. Fasting ≥85 mg/dL is not diagnostic. The accepted threshold is ≥95 mg/dL. A value of 85 mg/dL may be normal in pregnancy due to physiologic insulin resistance, and using it would lead to overdiagnosis and unnecessary interventions.
Take home points
- GDM diagnosis requires at least 2 abnormal values from the 100-gram OGTT.
- Diagnostic thresholds: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, 3-hour ≥140 mg/dL.
- Values below these thresholds are not diagnostic.
Practice Exercise 4
A nurse is assessing a patient with GDM for signs of preeclampsia. Which symptom should be reported immediately?
Explanation
Preeclampsia is a multisystem disorder of pregnancy characterized by hypertension, proteinuria, endothelial dysfunction, and organ ischemia. It typically occurs after 20 weeks gestation and may progress rapidly. Severe features include systolic blood pressure ≥160 mmHg, diastolic ≥110 mmHg, elevated liver enzymes, thrombocytopenia <100,000/mm³, and persistent epigastric or right upper quadrant pain due to hepatic involvement.
Rationale for correct answer
2. Epigastric pain in a patient with gestational diabetes mellitus (GDM) raises concern for hepatic capsular distension due to periportal necrosis, a hallmark of severe preeclampsia. This symptom reflects liver involvement and may precede HELLP syndrome. Immediate reporting is essential to prevent progression to eclampsia or placental abruption.
Rationale for incorrect answers
1. Increased appetite is not a feature of preeclampsia. It may occur in GDM due to fluctuating glucose levels or insulin adjustments but does not indicate organ dysfunction. It lacks correlation with vascular compromise or hepatic involvement.
3. Mild fatigue is nonspecific and common in pregnancy due to increased metabolic demand and hormonal shifts. It does not reflect end-organ damage or signal an acute complication. It lacks diagnostic specificity for preeclampsia.
4. Frequent urination is typical in pregnancy due to uterine pressure on the bladder and increased glomerular filtration rate. It is not associated with preeclampsia unless accompanied by oliguria or proteinuria. It does not indicate systemic compromise.
Take home points
- Epigastric pain in pregnancy may signal hepatic involvement in preeclampsia.
- GDM increases risk for preeclampsia due to vascular and metabolic stress.
- Fatigue and urinary frequency are common but nonspecific pregnancy symptoms.
- HELLP syndrome may present with epigastric pain before lab abnormalities.
A client with Gestational Diabetes Mellitus is found to have polyhydramnios during a routine ultrasound. This finding increases her risk for which of the following obstetric complications?
Explanation
Polyhydramnios is defined as excessive amniotic fluid volume, typically an amniotic fluid index (AFI) >24 cm or single deepest pocket >8 cm. It is associated with fetal anomalies, maternal diabetes, uterine overdistension, and preterm labor. The excess fluid stretches the uterus, triggering contractions and cervical changes. It may also cause malpresentation and cord prolapse. Common causes include fetal anencephaly, esophageal atresia, and uncontrolled hyperglycemia.
Rationale for correct answer
2. Preterm labor is a direct consequence of uterine overdistension caused by excessive amniotic fluid. The stretched myometrium increases contractility, leading to cervical effacement and dilation before 37 weeks. In gestational diabetes mellitus, poor glycemic control contributes to fetal polyuria and fluid accumulation, increasing this risk.
Rationale for incorrect answers
1. Oligohydramnios is the opposite of polyhydramnios and involves reduced amniotic fluid volume (AFI <5 cm). It is associated with placental insufficiency and renal anomalies, not maternal diabetes. It does not result from excess fluid and is not a complication of polyhydramnios.
3. Placenta previa involves abnormal placental implantation over the cervical os. It is linked to prior cesarean delivery and multiparity, not fluid volume abnormalities. Polyhydramnios does not affect placental location or increase risk for previa.
4. Vasa previa is a rare condition where fetal vessels traverse the membranes over the cervical os. It is associated with velamentous cord insertion and accessory lobes, not polyhydramnios. Excess fluid does not predispose to abnormal vessel placement.
Take home points
- Polyhydramnios increases uterine stretch and risk for preterm labor.
- Gestational diabetes causes fetal polyuria, contributing to fluid excess.
- Oligohydramnios is associated with placental insufficiency and renal defects.
- Vasa previa and placenta previa are structural, not fluid-related complications.
Which fetal complication is associated with uncontrolled gestational diabetes?
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically after 24 weeks due to increased placental hormones, insulin resistance, maternal hyperglycemia, and fetal hyperinsulinemia. Uncontrolled GDM leads to excessive glucose transfer across the placenta, stimulating fetal pancreatic beta cells to produce insulin. This results in increased fat deposition, organomegaly, and accelerated growth. Fetal complications include macrosomia, shoulder dystocia, neonatal hypoglycemia, and polyhydramnios. Fetal weight >4,000 g or >90th percentile defines macrosomia.
Rationale for correct answer
2. Macrosomia occurs due to fetal hyperinsulinemia secondary to maternal hyperglycemia. Insulin acts as a growth hormone, promoting adipose tissue accumulation and somatic overgrowth. This increases risk for birth trauma, shoulder dystocia, and cesarean delivery. The question stem specifies uncontrolled GDM, which directly drives this pathophysiology.
Rationale for incorrect answers
1. Microsomia refers to fetal growth restriction, typically <10th percentile for gestational age. It results from placental insufficiency, hypertensive disorders, or maternal malnutrition. It is not associated with hyperglycemia or insulin excess. GDM promotes overgrowth, not restriction.
3. Oligohydramnios is defined as amniotic fluid index <5 cm. It is linked to renal agenesis, uteroplacental insufficiency, and post-term pregnancy. GDM more commonly causes polyhydramnios due to fetal polyuria. It does not reduce fluid volume unless vascular compromise occurs.
4. Anemia in the fetus is caused by alloimmunization, parvovirus B19, or fetal-maternal hemorrhage. GDM does not impair erythropoiesis or cause hemolysis. There is no mechanism linking maternal hyperglycemia to fetal anemia.
Take home points
- Macrosomia results from fetal hyperinsulinemia due to maternal hyperglycemia.
- GDM increases risk for shoulder dystocia and birth trauma.
- Oligohydramnios and microsomia are linked to placental insufficiency, not GDM.
- Fetal anemia is unrelated to maternal glucose levels.
A nurse is providing education to a pregnant client newly diagnosed with Gestational Diabetes Mellitus regarding potential fetal complications. Which of the following complications should the nurse include? Select all that apply
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically after 24 weeks gestation due to placental hormones, insulin resistance, maternal hyperglycemia, and fetal hyperinsulinemia. Excess maternal glucose crosses the placenta, stimulating fetal pancreatic beta cells to produce insulin. This leads to macrosomia, neonatal hypoglycemia, and delayed pulmonary maturation. GDM increases risk for shoulder dystocia, birth trauma, and neonatal metabolic instability.
Rationale for correct answers
1. Macrosomia results from fetal hyperinsulinemia triggered by maternal hyperglycemia. Insulin acts as a growth hormone, promoting adipose tissue deposition and organomegaly. Fetal weight >4,000 g or >90th percentile defines macrosomia. This increases risk for shoulder dystocia and cesarean delivery.
2. Neonatal hypoglycemia occurs due to persistent fetal insulin secretion after birth, in the absence of maternal glucose supply. Blood glucose <40 mg/dL in the first 24 hours is diagnostic. Symptoms include jitteriness, apnea, and poor feeding. Early feeding and glucose monitoring are essential.
3. Respiratory distress syndrome (RDS) is more common in infants of diabetic mothers due to delayed surfactant synthesis. Hyperinsulinemia inhibits type II pneumocyte maturation. RDS presents with grunting, nasal flaring, and retractions. Risk is highest before 37 weeks gestation.
Rationale for incorrect answers
4. Microcephaly is defined as head circumference <2 standard deviations below mean for gestational age. It results from genetic syndromes, intrauterine infections, or teratogen exposure. GDM does not impair brain growth or cause cranial hypoplasia. It is not a recognized complication of maternal hyperglycemia.
5. Spina bifida is a neural tube defect caused by folate deficiency, valproate exposure, or genetic mutations. It occurs during early embryogenesis before 6 weeks gestation. GDM develops later and does not affect neural tube closure. There is no mechanistic link between GDM and spina bifida.
Take home points
- GDM causes fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.
- RDS results from delayed surfactant production due to insulin interference.
- Microcephaly and spina bifida are not complications of GDM.
- Early glucose control reduces risk of fetal metabolic and respiratory complications.
Which of the following are maternal complications of GDM? Select all that apply
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically after 24 weeks gestation due to placental hormones, insulin resistance, maternal hyperglycemia, and beta-cell dysfunction. GDM increases maternal risk for preeclampsia, operative delivery, and future metabolic disease. Poor glycemic control leads to endothelial injury, exaggerated inflammatory response, and vascular compromise. Long-term risks include progression to type 2 diabetes mellitus (T2DM), especially if fasting glucose exceeds 95 mg/dL or postpartum glucose remains elevated.
Rationale for correct answers
1. Preeclampsia is more common in GDM due to endothelial dysfunction and vascular inflammation. Hyperglycemia promotes oxidative stress and impairs nitric oxide-mediated vasodilation. This leads to hypertension, proteinuria, and organ ischemia. Risk increases with poor glycemic control and obesity.
2. Increased risk of cesarean delivery results from macrosomia and labor dystocia. Excess fetal growth due to maternal hyperglycemia leads to shoulder dystocia and failed labor progression. Cesarean rates are higher in GDM pregnancies, especially when fetal weight exceeds 4,000 g.
4. Type 2 diabetes risk postpartum is elevated due to persistent insulin resistance and beta-cell dysfunction. Up to 50% of women with GDM develop T2DM within 10 years. Risk increases with obesity, family history, and elevated postpartum glucose. Annual screening is recommended.
Rationale for incorrect answers
3. Hypoglycemia is not a typical maternal complication of GDM. It occurs in type 1 diabetes due to insulin overdose or missed meals. In GDM, maternal glucose levels are elevated, and insulin therapy is titrated to avoid hypoglycemia. It is rare unless overtreatment occurs.
5. Chronic renal failure is not directly caused by GDM. It results from long-standing hypertension, diabetic nephropathy, or glomerular disease. GDM is transient and typically resolves postpartum. Renal failure may occur in preexisting diabetes but is not a complication of gestational diabetes alone.
Take home points
- GDM increases risk for preeclampsia due to endothelial dysfunction.
- Cesarean delivery is more likely due to macrosomia and labor complications.
- Women with GDM have high lifetime risk for type 2 diabetes.
- Hypoglycemia and renal failure are not typical maternal complications of GDM.
Practice Exercise 5
A nurse is educating a patient with GDM about medical nutrition therapy. What percentage of total calories should come from carbohydrates?
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy. It results from insulin resistance, placental hormones, and increased metabolic demands. GDM typically emerges in the second trimester due to rising levels of human placental lactogen, cortisol, and progesterone. Symptoms include polyuria, polydipsia, and fatigue. Diagnosis is confirmed via a 1-hour glucose challenge test ≥140 mg/dL or a 3-hour oral glucose tolerance test. Management includes dietary control, exercise, and insulin if needed. Medical nutrition therapy aims to maintain fasting glucose <95 mg/dL and 1-hour postprandial <140 mg/dL.
Rationale for correct answer
2. Carbohydrates should provide 40–50% of total daily calories in GDM to ensure adequate fetal growth while minimizing postprandial glucose spikes. This range supports glycemic control and prevents ketosis. Complex carbohydrates with low glycemic index are preferred. The question stem focuses on medical nutrition therapy, which prioritizes balanced macronutrient distribution.
Rationale for incorrect answers
1. A carbohydrate intake of 20–30% is too low and may lead to ketosis and inadequate caloric intake. Ketone production from fat metabolism can impair fetal neurodevelopment. This range does not meet the energy demands of pregnancy and contradicts standard GDM dietary guidelines.
3. A carbohydrate intake of 60–70% is excessive and increases the risk of postprandial hyperglycemia. High carbohydrate loads can overwhelm insulin response, especially in insulin-resistant states like GDM. This range is not recommended as it compromises glycemic control.
4. A carbohydrate intake of 70–80% is dangerously high and leads to poor glucose regulation. It promotes frequent hyperglycemic episodes and increases the likelihood of fetal macrosomia and neonatal hypoglycemia. This percentage is inconsistent with evidence-based GDM nutrition therapy.
Take home points
- GDM requires controlled carbohydrate intake to prevent hyperglycemia and ketosis.
- Carbohydrates should comprise 40–50% of total daily calories in GDM.
- Excessive carbohydrate intake worsens glycemic control and fetal outcomes.
- Low carbohydrate intake risks ketosis and inadequate fetal nutrition.
Which of the following is considered the gold standard pharmacological treatment for Gestational Diabetes Mellitus due to its efficacy and minimal placental transfer?
Explanation
Gestational diabetes mellitus (GDM) is a pregnancy-induced glucose intolerance caused by insulin resistance, placental hormones, and increased metabolic demands. Human placental lactogen, cortisol, and progesterone antagonize insulin, especially in the second and third trimesters. GDM presents with hyperglycemia, polyuria, and fatigue. Diagnosis is confirmed via a 1-hour glucose challenge ≥140 mg/dL or a 3-hour oral glucose tolerance test. Management includes dietary control, exercise, and pharmacologic therapy when glucose targets are not met. Fasting glucose should be <95 mg/dL and 1-hour postprandial <140 mg/dL.
Rationale for correct answer
3. Insulin is the gold standard pharmacologic treatment for GDM due to its high efficacy and minimal placental transfer. It does not cross the placenta in significant amounts, making it safe for fetal development. It allows precise titration to maintain euglycemia and prevents complications such as macrosomia and neonatal hypoglycemia.
Rationale for incorrect answers
1. Glyburide is a sulfonylurea that stimulates pancreatic insulin release but crosses the placenta and may cause neonatal hypoglycemia. Its pharmacokinetics are unpredictable in pregnancy, and it has a higher failure rate in achieving glycemic targets compared to insulin.
2. Metformin improves insulin sensitivity and reduces hepatic glucose output but crosses the placenta and accumulates in fetal tissues. Long-term fetal safety data are limited. It is less effective than insulin in controlling postprandial spikes and is often used as adjunct therapy, not first-line.
4. Acarbose is an alpha-glucosidase inhibitor that delays carbohydrate absorption in the intestine. It has minimal systemic absorption but is not widely studied in pregnancy and lacks robust data on fetal safety and efficacy. It is not recommended as first-line therapy for GDM.
Take home points
- Insulin is the preferred pharmacologic agent for GDM due to minimal placental transfer.
- Oral agents like glyburide and metformin cross the placenta and have variable efficacy.
- GDM arises from placental hormone-induced insulin resistance.
- Tight glycemic control reduces fetal and maternal complications.
A nurse is instructing a patient on self-monitoring blood glucose. How many times daily should glucose levels be checked?
Explanation
Self-monitoring of blood glucose (SMBG) is essential in diabetes management to guide therapy and prevent complications. It enables detection of hyperglycemia, hypoglycemia, and postprandial spikes. In gestational diabetes mellitus, SMBG helps maintain glucose targets: fasting <95 mg/dL, 1-hour postprandial <140 mg/dL. Accurate monitoring requires checking at multiple time points to assess glycemic variability and treatment efficacy. Meal timing, insulin use, and glycemic targets determine frequency. SMBG data informs adjustments in diet, activity, and medication.
Rationale for correct answer
4. Four times daily monitoring includes fasting and postprandial checks after each meal. This pattern captures glucose fluctuations and ensures tight control. It aligns with clinical guidelines for gestational diabetes and insulin-treated patients. Frequent monitoring reduces risk of fetal macrosomia and maternal complications.
Rationale for incorrect answers
1. Once daily monitoring is insufficient to detect glycemic variability. It misses postprandial spikes and fasting trends. This frequency cannot guide therapy adjustments or ensure glucose targets are met.
2. Twice daily checks may capture fasting and one postprandial value but miss meal-related excursions. It underestimates hyperglycemia risk and limits therapeutic precision.
3. Three times daily omits one postprandial value, reducing detection of post-meal hyperglycemia. It may be used in stable patients but is inadequate for initial management or insulin therapy.
Take home points
- SMBG in gestational diabetes should be done four times daily.
- Monitoring includes fasting and postprandial values.
- Less frequent checks miss glycemic excursions and compromise control.
Which of the following are components of medical nutrition therapy for GDM? Select all that apply
Explanation
Medical nutrition therapy in gestational diabetes mellitus (GDM) aims to maintain maternal euglycemia and prevent fetal complications. It relies on macronutrient balance, glycemic control, and individualized caloric needs. GDM results from placental hormone-induced insulin resistance, especially in the second and third trimesters. Symptoms include polyuria, polydipsia, and fatigue. Fasting glucose should be <95 mg/dL and 1-hour postprandial <140 mg/dL. Nutrition therapy must avoid ketosis, ensure adequate fetal growth, and prevent macrosomia.
Rationale for correct answers
1. Carbohydrates should provide 40–50% of total daily calories to support fetal growth while minimizing postprandial hyperglycemia. This proportion ensures adequate energy without triggering excessive glucose excursions. Complex carbohydrates with low glycemic index are preferred.
3. Distributing intake across three meals and 2–3 snacks stabilizes blood glucose levels and prevents ketosis. This pattern reduces glycemic variability and supports consistent insulin response. It also prevents prolonged fasting, which can elevate ketone levels.
5. Caloric intake must be based on prepregnancy BMI to avoid excessive weight gain and fetal macrosomia. For BMI <18.5, recommend 30–35 kcal/kg/day; for BMI 18.5–24.9, 25–30 kcal/kg/day; for BMI >25, 20–25 kcal/kg/day. This ensures individualized energy needs are met.
Rationale for incorrect answers
2. Emphasis on simple sugars is contraindicated in GDM due to rapid glucose absorption and high glycemic index. Simple sugars cause postprandial spikes and compromise glycemic control. Medical nutrition therapy prioritizes complex carbohydrates and fiber-rich foods.
4. A high-fat diet increases insulin resistance and promotes dyslipidemia. Saturated fats impair glucose metabolism and elevate triglycerides. GDM nutrition should include moderate fat intake (25–35% of total calories), emphasizing unsaturated fats.
Take home points
- Carbohydrates should comprise 40–50% of total calories in GDM.
- Meals and snacks must be evenly distributed to prevent ketosis.
- Caloric needs are based on prepregnancy BMI to avoid fetal overgrowth.
- Avoid simple sugars and high-fat diets to maintain glycemic control.
Which of the following are important teaching points for insulin administration? Select all that apply
Explanation
Insulin administration requires precise technique and patient education to ensure therapeutic efficacy and prevent complications. Proper site rotation, subcutaneous technique, hypoglycemia recognition, and safe disposal are essential. Lipodystrophy occurs due to repeated injections at the same site. Hypoglycemia presents with tremors, sweating, confusion, and glucose <70 mg/dL. Injection technique affects absorption rate and insulin kinetics. Insulin vials should be stored at room temperature for up to 28 days, not indefinitely.
Rationale for correct answers
1. Rotating injection sites prevents lipodystrophy and ensures consistent absorption. Repeated injections at the same site cause fatty tissue changes, leading to erratic insulin uptake. Teaching patients to rotate sites systematically improves glycemic control and reduces tissue damage.
4. Gently pinching the skin before injecting ensures subcutaneous delivery and avoids intramuscular administration. Intramuscular injections can alter insulin absorption rates, increasing the risk of hypoglycemia. Pinching lifts the fat layer, optimizing depth and reducing pain.
5. Recognizing and treating hypoglycemia is critical in insulin therapy. Symptoms include sweating, tremors, confusion, and palpitations. Immediate treatment involves consuming 15 g of fast-acting carbohydrates like glucose tablets or juice. Delayed recognition can lead to seizures or coma.
Rationale for incorrect answers
2. Insulin vials cannot be stored at room temperature indefinitely. Room temperature storage is acceptable for only 28 days. Beyond this, insulin loses potency and may become ineffective. Refrigeration is necessary for unopened vials to maintain stability.
3. Disposing of needles in regular trash is unsafe and violates biohazard protocols. Used needles must be placed in puncture-resistant sharps containers to prevent injury and contamination. Improper disposal poses risks to sanitation workers and the public.
Take home points
- Rotate insulin injection sites to prevent lipodystrophy and ensure consistent absorption.
- Pinch skin before injecting to ensure proper subcutaneous delivery and avoid intramuscular injection.
- Hypoglycemia must be recognized early and treated with fast-acting carbohydrates.
Practice Exercise 6
What is the purpose of weekly nonstress tests in women with GDM requiring insulin?
Explanation
Gestational diabetes mellitus (GDM) requiring insulin increases the risk of placental insufficiency, fetal hypoxia, macrosomia, and stillbirth. Weekly nonstress tests (NSTs) assess fetal autonomic function and oxygenation by evaluating heart rate accelerations in response to fetal movement. A reactive NST shows ≥2 accelerations of ≥15 bpm lasting ≥15 seconds within 20 minutes. Insulin use indicates higher risk, necessitating close fetal surveillance.
Rationale for correct answer
2. Weekly nonstress tests are used to monitor fetal well-being in pregnancies complicated by GDM requiring insulin. Insulin-dependent GDM increases the risk of placental insufficiency, which can lead to fetal hypoxia. NSTs detect fetal heart rate accelerations, indicating intact autonomic and oxygenation status. A reactive NST confirms adequate fetal oxygenation.
Rationale for incorrect answers
1. Nonstress tests do not assess maternal glucose levels. Glucose monitoring is done via capillary blood glucose testing or continuous glucose monitoring systems. NSTs are fetal assessments and do not provide any data on maternal glycemic control.
3. Amniotic fluid volume is measured using ultrasound, specifically the amniotic fluid index (AFI) or single deepest pocket. NSTs do not visualize or quantify fluid levels. Oligohydramnios or polyhydramnios requires sonographic evaluation, not cardiotocography.
4. Placental size is evaluated via ultrasound imaging, not NST. NSTs assess fetal heart rate patterns, not anatomical features. Placental morphology, calcifications, or size abnormalities require direct imaging for assessment.
Take home points
- NSTs assess fetal heart rate response to movement, indicating oxygenation and autonomic integrity.
- Insulin-dependent GDM increases risk of fetal hypoxia and stillbirth, requiring weekly NSTs.
- NSTs do not evaluate maternal glucose, amniotic fluid volume, or placental size.
- Ultrasound is the modality for anatomical and fluid assessments in pregnancy.
Which antenatal test primarily monitors fetal heart rate accelerations in response to fetal movement?
Explanation
Non-stress test (NST) is a fetal surveillance tool used to assess autonomic function, oxygenation, cardiac reactivity, and neurologic integrity. It evaluates fetal heart rate accelerations in response to spontaneous movement. A reactive NST shows ≥2 accelerations of ≥15 bpm lasting ≥15 seconds within 20 minutes. It is noninvasive and used in high-risk pregnancies including diabetes, hypertension, and IUGR. NST does not require uterine contractions and reflects intact fetal sympathetic and parasympathetic systems.
Rationale for correct answer
3. Non-stress test directly monitors fetal heart rate accelerations in response to fetal movement. It is the primary tool for assessing fetal oxygenation and neurologic status. A reactive NST indicates intact autonomic regulation and adequate placental perfusion. It is performed without inducing contractions and is repeated weekly or biweekly in high-risk pregnancies.
Rationale for incorrect answers
1. Biophysical profile includes NST as one of its 5 components but does not primarily monitor heart rate accelerations. It evaluates fetal tone, movement, breathing, amniotic fluid, and NST. The composite score reflects overall fetal well-being, not just cardiac reactivity.
2. Contraction stress test evaluates fetal heart rate response to induced uterine contractions, not spontaneous movement. It assesses placental reserve and risk of late decelerations. CST is used when NST is nonreactive and requires oxytocin or nipple stimulation to induce contractions.
4. Amniocentesis is an invasive test used to assess fetal genetics, lung maturity, or infection, not heart rate. It involves aspiration of amniotic fluid under ultrasound guidance. It does not provide any data on fetal movement or cardiac accelerations.
Take home points
- NST is the primary test for assessing fetal heart rate accelerations in response to movement.
- Reactive NST indicates intact autonomic function and adequate oxygenation.
- CST evaluates fetal response to contractions, not spontaneous movement.
- BPP includes NST but is a composite test assessing multiple fetal parameters.
What is the purpose of a biophysical profile in GDM pregnancies?
Explanation
Biophysical profile (BPP) is a fetal surveillance tool used in high-risk pregnancies such as gestational diabetes mellitus (GDM) to assess fetal well-being, oxygenation, neurologic integrity, and placental sufficiency. It combines ultrasound evaluation of fetal movement, tone, breathing, and amniotic fluid volume with a non-stress test. Each component is scored 0 or 2, with a maximum score of 10. A score of 8–10 is reassuring; ≤6 may indicate hypoxia or placental dysfunction.
Rationale for correct answer
2. Biophysical profile assesses fetal growth and well-being in GDM pregnancies. It evaluates fetal movement, tone, breathing, amniotic fluid volume, and heart rate reactivity. These parameters reflect fetal oxygenation and neurologic status. GDM increases risk of placental insufficiency and fetal hypoxia, making BPP essential for monitoring.
Rationale for incorrect answers
1. BPP does not measure maternal glucose levels. Glucose monitoring is done via capillary blood glucose testing or continuous glucose monitoring. BPP is a fetal assessment tool and does not provide maternal metabolic data.
3. BPP does not evaluate placental hormone levels. Hormonal assays such as human placental lactogen or estriol are separate tests. BPP assesses fetal behavior and amniotic fluid, not endocrine function.
4. BPP does not monitor maternal blood pressure. Blood pressure is assessed using sphygmomanometry and is part of maternal surveillance. BPP is focused on fetal parameters and does not include maternal hemodynamics.
Take home points
- BPP assesses fetal well-being using ultrasound and NST components.
- GDM increases risk of fetal hypoxia, making BPP a key surveillance tool.
- BPP does not measure maternal glucose, blood pressure, or placental hormones.
- A score ≤6 may indicate compromised fetal oxygenation and require intervention.
Which of the following are components assessed in a Biophysical Profile (BPP) for fetal surveillance in a client with Gestational Diabetes Mellitus? Select all that apply
Explanation
Biophysical profile (BPP) is a fetal surveillance tool used in high-risk pregnancies such as gestational diabetes mellitus (GDM) to evaluate oxygenation, neurologic integrity, placental function, and fetal well-being. It includes 5 components: fetal breathing, movement, tone, amniotic fluid volume, and non-stress test (NST). Each component is scored 0 or 2, with a total score out of 10. A score of 8–10 is reassuring; ≤6 may indicate fetal compromise. GDM increases risk of placental insufficiency and fetal hypoxia, making BPP essential.
Rationale for correct answers
1. Fetal heart rate accelerations are assessed via non-stress test, which is one of the 5 components of BPP. It evaluates autonomic function and oxygenation by detecting accelerations in response to fetal movement. A reactive NST confirms intact neurologic status.
2. Fetal breathing movements are assessed using ultrasound during BPP. Presence of rhythmic diaphragmatic movements lasting ≥30 seconds within 30 minutes indicates intact central nervous system function and adequate oxygenation.
4. Amniotic fluid volume is measured by ultrasound, either via amniotic fluid index (AFI) or single deepest pocket. Adequate fluid reflects placental perfusion and fetal renal function. Oligohydramnios may indicate chronic hypoxia or placental insufficiency.
Rationale for incorrect answers
3. Maternal blood pressure is not part of BPP. It is monitored separately using sphygmomanometry and is part of maternal surveillance. BPP focuses exclusively on fetal parameters and does not include maternal hemodynamics.
5. Fetal kidney function is not directly assessed in BPP. While amniotic fluid reflects fetal urine output, BPP does not evaluate renal anatomy or function. Detailed renal assessment requires targeted ultrasound or biochemical testing.
Take home points
- BPP includes NST, fetal breathing, movement, tone, and amniotic fluid volume.
- GDM increases risk of fetal hypoxia, making BPP essential for surveillance.
- Maternal blood pressure and fetal kidney function are not components of BPP.
- Amniotic fluid volume reflects placental perfusion and fetal renal output.
Which of the following are included in fetal surveillance for GDM? Select all that apply
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy. It results from placental hormones, insulin resistance, and maternal metabolic stress, typically manifesting in the second or third trimester. GDM increases risk for macrosomia, polyhydramnios, and stillbirth. Fetal surveillance aims to detect hypoxia, growth abnormalities, and placental insufficiency. Ultrasound is used to monitor estimated fetal weight, with macrosomia defined as >4,000 g. Nonstress tests assess fetal heart rate reactivity, while biophysical profiles combine ultrasound and NST data to evaluate fetal well-being. Surveillance begins around 32 weeks if GDM is poorly controlled or insulin-dependent.
Rationale for correct answers
1. Nonstress tests are used to assess fetal oxygenation and autonomic function by evaluating heart rate patterns in response to fetal movement. In GDM, especially when insulin is used, NSTs are initiated weekly or biweekly from 32 weeks to detect early signs of hypoxia.
2. Biophysical profiles combine ultrasound parameters (fetal tone, movement, breathing, amniotic fluid) with NST results to quantify fetal well-being. In GDM, BPPs help detect placental insufficiency and guide timing of delivery, especially when NSTs are nonreactive or amniotic fluid is reduced.
4. Ultrasound for fetal growth is essential in GDM to monitor for macrosomia and growth restriction. Serial ultrasounds are performed every 3 to 4 weeks to track estimated fetal weight, abdominal circumference, and amniotic fluid index.
Rationale for incorrect answers
3. Maternal glucose monitoring is a maternal surveillance tool, not fetal surveillance. It guides insulin therapy and dietary adjustments but does not directly assess fetal status. While critical for glycemic control, it does not evaluate fetal oxygenation or growth.
5. Maternal blood pressure checks are part of maternal monitoring, especially to detect preeclampsia, which may coexist with GDM. However, blood pressure measurement does not provide any direct information about fetal well-being or intrauterine status.
Take home points
- Fetal surveillance in GDM includes NSTs, BPPs, and ultrasound for growth.
- Maternal glucose and blood pressure monitoring are not fetal surveillance tools.
- Macrosomia and stillbirth risk increase with poorly controlled GDM.
- Surveillance typically begins at 32 weeks in insulin-dependent GDM.
Practice Exercise 7
A nurse is educating a patient about hypoglycemia symptoms. Which symptom should the patient report?
Explanation
Hypoglycemia is a clinical state characterized by blood glucose levels falling below 70 mg/dL. It results from excessive insulin, missed meals, or increased physical activity. Neuroglycopenic symptoms include confusion, blurred vision, and seizures, while adrenergic symptoms include tremors, palpitations, and anxiety. Rapid onset, especially in insulin-treated patients, is common. Counterregulatory hormones like epinephrine trigger early warning signs.
Rationale for correct answer
2. Shakiness is a classic adrenergic response to falling glucose levels. The sympathetic nervous system releases epinephrine, causing tremors and palpitations. This symptom is often the earliest and most noticeable sign, especially in patients with intact autonomic function. The question stem asks what symptom should be reported, and shakiness directly reflects acute hypoglycemia.
Rationale for incorrect answers
1. Increased thirst is a hallmark of hyperglycemia, not hypoglycemia. Elevated serum glucose causes osmotic diuresis, leading to dehydration and polydipsia. In hypoglycemia, fluid balance is typically unaffected unless comorbid conditions exist. This symptom reflects high glucose, not low.
3. Weight gain is a chronic metabolic effect, not an acute symptom. It may occur with insulin therapy due to anabolic effects or reduced glycosuria, but it is not a warning sign of hypoglycemia. The question targets immediate symptoms, not long-term outcomes.
4. Constipation is unrelated to glucose levels. It may result from low fiber intake, dehydration, or medications like opioids, but it is not a neurogenic or adrenergic symptom of hypoglycemia. It lacks temporal association with glucose fluctuations.
Take home points
- Hypoglycemia presents with adrenergic and neuroglycopenic symptoms.
- Shakiness is an early adrenergic sign due to epinephrine release.
- Increased thirst is a symptom of hyperglycemia, not hypoglycemia.
- Weight gain and constipation are not acute indicators of low blood glucose.
A nurse is counseling a postpartum client with a history of Gestational Diabetes Mellitus about long-term health. What is the most important recommendation for preventing Type 2 Diabetes Mellitus?
Explanation
Gestational Diabetes Mellitus (GDM) is a glucose intolerance first recognized during pregnancy, typically resolving postpartum. However, it significantly increases the risk of Type 2 Diabetes Mellitus (T2DM) later in life. Insulin resistance, driven by placental hormones, is the core mechanism. Postpartum, pancreatic beta-cell dysfunction may persist, especially with obesity. Lifestyle modification is the most effective preventive strategy. Women with prior GDM have a 35%–60% chance of developing T2DM within 10 years.
Rationale for correct answer
2. Maintaining a healthy weight and engaging in regular physical activity directly improves insulin sensitivity and reduces visceral adiposity, both critical in preventing T2DM. Exercise enhances glucose uptake independent of insulin, while weight control reduces inflammatory cytokines that impair insulin action. This is the most evidence-based and sustainable recommendation for postpartum women with prior GDM.
Rationale for incorrect answers
1. Avoiding all carbohydrates indefinitely is neither practical nor physiologically sound. Carbohydrates are essential for glucose homeostasis and energy metabolism. The focus should be on complex carbohydrates with low glycemic index, not total elimination. Long-term restriction may lead to nutrient deficiencies and poor adherence.
3. Taking oral hypoglycemic agents for life is not recommended for prevention. These drugs are used for glycemic control, not risk reduction in normoglycemic individuals. Metformin may be considered in high-risk cases, but lifestyle changes remain first-line. Lifelong pharmacotherapy without indication exposes patients to unnecessary side effects.
4. Limiting fluid intake to prevent fluid retention is unrelated to diabetes prevention. Fluid retention is influenced by renal function and sodium balance, not glucose metabolism. In fact, adequate hydration supports renal glucose clearance and overall metabolic health. This recommendation lacks scientific basis in diabetes prevention.
Take home points
- Prior GDM increases future risk of Type 2 Diabetes Mellitus.
- Lifestyle changes are the most effective preventive strategy.
- Carbohydrate quality matters more than total avoidance.
- Oral hypoglycemics are not used for primary prevention in normoglycemic individuals.
A nurse is assessing a patient’s understanding of GDM. Which statement indicates a need for further education?
Explanation
Gestational Diabetes Mellitus (GDM) is a glucose intolerance first diagnosed during pregnancy, typically after 24 weeks gestation. It results from placental hormone-induced insulin resistance, especially human placental lactogen. Postprandial hyperglycemia is common and requires tight control to prevent fetal complications. Medical nutrition therapy, glucose monitoring, and exercise are first-line interventions. Insulin is added if targets are not met. Fasting glucose should be <95 mg/dL, 1-hour postprandial <140 mg/dL, and 2-hour <120 mg/dL.
Rationale for correct answer
2. The statement reflects a misunderstanding of dietary management in GDM. Even with insulin, excessive intake of simple sugars causes unpredictable glycemic excursions, increasing risk of macrosomia and neonatal hypoglycemia. Insulin dosing is calibrated to controlled intake, not unrestricted consumption. Patient education must emphasize carbohydrate quality and portion control.
Rationale for incorrect answers
1. Checking blood sugar four times daily is standard in GDM management. Typically, this includes fasting and postprandial readings to guide therapy. Frequent monitoring allows timely insulin adjustments and dietary modifications. This statement reflects accurate understanding.
3. Exercise improves insulin sensitivity and promotes glucose uptake by skeletal muscle. Moderate activity like walking after meals helps reduce postprandial spikes. This is a cornerstone of non-pharmacologic management and is correctly stated.
4. Neonates of mothers with GDM are at risk for hypoglycemia due to fetal hyperinsulinemia. Glucose checks post-delivery are essential to detect and treat early hypoglycemia. This reflects correct understanding of neonatal monitoring protocols.
Take home points
- GDM requires strict dietary control even when insulin is used.
- Blood glucose monitoring is essential for therapy adjustment.
- Exercise enhances insulin sensitivity and reduces postprandial glucose.
- Neonatal glucose checks are critical due to risk of hypoglycemia.
Which of the following are signs of hypoglycemia a nurse should teach a patient with GDM? Select all that apply
Explanation
Hypoglycemia is defined as a blood glucose level below 70 mg/dL and is a common complication in insulin-treated Gestational Diabetes Mellitus (GDM). It results from excess insulin, skipped meals, or increased physical activity. Adrenergic symptoms like shakiness and sweating occur due to epinephrine release, while neuroglycopenic symptoms like confusion arise from inadequate glucose supply to the brain. Rapid onset, especially in insulin-treated patients, is typical. Counterregulatory mechanisms attempt to restore glucose but may be impaired in pregnancy.
Rationale for correct answers
1. Shakiness is an adrenergic symptom triggered by epinephrine release in response to falling glucose. It reflects autonomic activation and is often the earliest sign. Patients with intact sympathetic response will notice tremors during hypoglycemia.
2. Sweating is another adrenergic symptom caused by sympathetic stimulation. It reflects autonomic dysregulation and typically accompanies tremors and palpitations. It is a reliable early warning sign in insulin-treated GDM.
4. Confusion is a neuroglycopenic symptom due to inadequate glucose delivery to the brain. It reflects cerebral dysfunction and may progress to seizures or coma if untreated. It is a late but critical sign of hypoglycemia.
Rationale for incorrect answers
3. Increased thirst is a symptom of hyperglycemia, not hypoglycemia. It results from osmotic diuresis due to elevated glucose levels causing dehydration. It does not occur in low glucose states and reflects a different pathophysiology.
5. Weight gain is a chronic metabolic effect, not an acute symptom. It may occur with insulin therapy due to anabolic effects, but it is unrelated to hypoglycemia. The question targets immediate signs, not long-term outcomes.
Take home points
- Hypoglycemia in GDM presents with adrenergic and neuroglycopenic symptoms.
- Shakiness and sweating are early adrenergic signs due to epinephrine.
- Confusion reflects cerebral glucose deprivation and is a late warning.
- Increased thirst and weight gain are not signs of hypoglycemia.
Which of the following are components of patient education for GDM? Select all that apply
Explanation
Gestational Diabetes Mellitus (GDM) is a glucose intolerance first diagnosed during pregnancy, typically after 24 weeks gestation. It results from placental hormone-induced insulin resistance, especially human placental lactogen. Postprandial hyperglycemia is common and requires tight control to prevent fetal complications. Medical nutrition therapy, glucose monitoring, and exercise are first-line interventions. Insulin is added if targets are not met. Fasting glucose should be <95 mg/dL, 1-hour postprandial <140 mg/dL, and 2-hour <120 mg/dL.
Rationale for correct answers
1. Understanding GDM implications is essential for informed decision-making. Patients must grasp risks such as macrosomia and neonatal hypoglycemia, and long-term maternal risk for Type 2 Diabetes. This knowledge supports adherence and postpartum follow-up.
2. Self-monitoring blood glucose techniques are foundational in GDM management. Patients must learn proper glucometer use and timing of checks—typically fasting and postprandial. Accurate monitoring guides therapy and prevents complications.
3. Adherence to medical nutrition therapy is the cornerstone of GDM control. Diet plans focus on carbohydrate distribution and glycemic index. Registered dietitian input ensures individualized, sustainable plans that stabilize glucose levels.
5. Recognizing hypoglycemia symptoms is critical, especially for insulin-treated patients. Symptoms like shakiness and confusion must prompt immediate glucose intake. Education prevents severe neuroglycopenic events and supports safe insulin use.
Rationale for incorrect answer
4. Avoiding all physical activity is incorrect and harmful. Moderate exercise improves insulin sensitivity and reduces postprandial glucose. Walking after meals is recommended. Sedentary behavior worsens glycemic control and increases insulin requirements.
Take home points
- GDM education includes understanding risks, monitoring, diet, and symptom recognition.
- Exercise is beneficial, not contraindicated, in GDM.
- Hypoglycemia awareness is vital for insulin-treated patients.
- Nutrition therapy focuses on carbohydrate quality and timing.
Comprehensive Questions
Which hormone contributes significantly to insulin resistance in gestational diabetes?
Explanation
Gestational diabetes is a form of glucose intolerance that develops during pregnancy due to hormonal changes, insulin resistance, placental factors, and metabolic stress. Human placental lactogen, cortisol, progesterone, and growth hormone antagonize insulin action. Insulin resistance peaks in the third trimester. Fasting glucose should remain below 95 mg/dL, and 2-hour postprandial below 120 mg/dL. Risk factors include obesity, polycystic ovarian syndrome, and family history of type 2 diabetes. Complications include macrosomia, neonatal hypoglycemia, and preeclampsia.
Rationale for correct answer
2. Human placental lactogen (hPL) is secreted by the syncytiotrophoblast and rises progressively during pregnancy. It has anti-insulin and lipolytic effects, increasing maternal insulin resistance to ensure glucose availability for the fetus. Its peak action in the third trimester correlates with the onset of gestational diabetes. The question asks for the hormone that contributes significantly to insulin resistance, making hPL the correct answer.
Rationale for incorrect answers
1. Insulin is a regulatory hormone produced by pancreatic beta cells. It lowers blood glucose by promoting cellular uptake and glycogenesis. It does not cause insulin resistance; rather, resistance occurs when cells fail to respond to insulin. In gestational diabetes, insulin secretion may increase to compensate, but the hormone itself is not the cause of resistance.
3. Estrogen levels rise during pregnancy and support uterine growth and vascularization. While estrogen has mild antagonistic effects on insulin, it does not significantly contribute to gestational insulin resistance. Its role is more prominent in modulating lipid metabolism and increasing hepatic protein synthesis, not in impairing glucose uptake.
4. Thyroxine (T4) is a thyroid hormone involved in basal metabolic rate regulation. It increases glucose absorption from the gut and enhances insulin degradation but does not directly induce insulin resistance. Thyroid dysfunction may affect glucose metabolism, but thyroxine is not a major contributor to gestational diabetes pathophysiology.
Take home points
- Human placental lactogen is the primary hormone causing insulin resistance in pregnancy.
- Gestational diabetes peaks in the third trimester due to placental hormone effects.
- Estrogen and thyroxine do not significantly impair insulin action.
- Insulin resistance in pregnancy is a physiological adaptation for fetal glucose supply.
Which ethnicity is at higher risk for developing gestational diabetes?
Explanation
Gestational diabetes mellitus (GDM) is a pregnancy-induced glucose intolerance caused by insulin resistance, placental hormones, beta-cell dysfunction, and ethnic predisposition. Human placental lactogen, cortisol, and progesterone impair insulin action, peaking in the third trimester. Fasting glucose should be <95 mg/dL and 2-hour postprandial <120 mg/dL. Ethnic groups with higher risk include Hispanic, Asian, Native American, and African American populations. GDM increases risk of macrosomia, neonatal hypoglycemia, and future type 2 diabetes.
Rationale for correct answer
2. Hispanic women have a significantly higher prevalence of GDM due to genetic susceptibility and metabolic risk factors. Studies show increased insulin resistance and reduced beta-cell compensation in this group. The question asks for the ethnicity with higher risk, and Hispanic populations consistently show elevated GDM incidence compared to non-Hispanic whites.
Rationale for incorrect answers
1. Caucasian women, particularly non-Hispanic whites, have a lower incidence of GDM. Their metabolic profile shows better insulin sensitivity and beta-cell compensation. While GDM can occur in any group, Caucasians are not considered high-risk compared to Hispanic, Asian, or African American populations.
3. Scandinavian populations have one of the lowest GDM rates globally. Their genetic background and dietary patterns contribute to lower insulin resistance. Population-based studies from Norway and Sweden confirm reduced GDM prevalence compared to multiethnic cohorts.
4. Irish ethnicity does not show elevated GDM risk in epidemiological studies. Their glucose tolerance and pregnancy outcomes align more closely with other Northern European groups. No significant data supports Irish women being at higher risk than Hispanic or Asian populations.
Take home points
- Hispanic ethnicity is a major risk factor for gestational diabetes.
- Scandinavian and Irish populations have lower GDM prevalence.
- Ethnic background influences insulin resistance and beta-cell function.
- GDM screening should be tailored to high-risk ethnic groups.
The nurse is reviewing the medical history of a pregnant client. Which of the following findings would indicate a high risk for Gestational Diabetes Mellitus?
Explanation
Gestational diabetes mellitus (GDM) is a pregnancy-induced glucose intolerance caused by insulin resistance, placental hormones, beta-cell dysfunction, and metabolic risk factors. It typically develops in the second or third trimester when insulin resistance peaks due to human placental lactogen, cortisol, and progesterone. Fasting glucose should be <95 mg/dL and 2-hour postprandial <120 mg/dL. Risk factors include obesity, prior macrosomic delivery (>4 kg), family history of type 2 diabetes, and Polycystic Ovary Syndrome (PCOS). PCOS contributes to insulin resistance even before pregnancy, increasing GDM risk.
Rationale for correct answer
3. Polycystic Ovary Syndrome is a known endocrine disorder associated with hyperandrogenism and insulin resistance. Women with PCOS have impaired glucose metabolism and reduced beta-cell compensation, making them highly susceptible to GDM. The presence of PCOS in the medical history directly increases the risk of gestational diabetes.
Rationale for incorrect answers
1. A history of delivering a 7-pound infant does not meet the threshold for macrosomia, which is defined as birth weight >4,000 g (8.8 pounds). Macrosomia is a clinical marker of prior glucose intolerance, but 7 pounds is within normal range and does not indicate elevated GDM risk.
2. A pre-pregnancy BMI of 22 kg/m² is within the normal weight range (18.5–24.9 kg/m²). Obesity (BMI ≥30 kg/m²) is a significant risk factor for GDM due to increased insulin resistance. A BMI of 22 does not confer elevated risk and reflects healthy metabolic status.
4. Maternal age of 20 years is not considered a risk factor for GDM. Advanced maternal age (>35 years) is associated with increased insulin resistance and beta-cell decline. Younger age groups typically have lower metabolic risk and better glucose tolerance.
Take home points
- PCOS significantly increases risk for gestational diabetes due to insulin resistance.
- Normal BMI and younger maternal age are not risk factors for GDM.
- Macrosomia is defined as birth weight >4,000 g, not 7 pounds.
- GDM screening should consider endocrine and metabolic history.
Which diagnostic threshold for the 100-gram, 3-hour OGTT indicates gestational diabetes if exceeded?
Explanation
Gestational diabetes mellitus (GDM) is diagnosed using the oral glucose tolerance test (OGTT), which evaluates glucose metabolism, insulin resistance, placental hormone effects, and beta-cell function. The 100-gram, 3-hour OGTT is performed after an 8–14 hour fast and requires unrestricted carbohydrate intake for 3 days prior. Diagnostic thresholds are: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, and 3-hour ≥140 mg/dL. GDM is confirmed if 2 or more values meet or exceed these thresholds. GDM increases risk of macrosomia, neonatal hypoglycemia, and future type 2 diabetes.
Rationale for correct answer
3. The 2-hour threshold for the 100-gram OGTT is 155 mg/dL. Exceeding this value indicates impaired glucose clearance and insulin resistance. The 2-hour value reflects postprandial glucose handling and is a critical diagnostic point. The question asks which threshold indicates GDM if exceeded, and 155 mg/dL is the correct 2-hour cutoff.
Rationale for incorrect answers
1. Fasting threshold for GDM diagnosis is ≥95 mg/dL, not 85 mg/dL. A value of 85 mg/dL is within normal fasting range and does not meet diagnostic criteria. Fasting glucose reflects basal insulin activity and hepatic glucose output, but 85 mg/dL is below the required threshold.
2. The 1-hour threshold is ≥180 mg/dL, not 170 mg/dL. A 1-hour value of 170 mg/dL is elevated but not diagnostic. The 1-hour reading assesses early insulin response and glucose absorption, but 170 mg/dL falls short of the diagnostic cutoff.
4. The 3-hour threshold is ≥140 mg/dL, not 130 mg/dL. A value of 130 mg/dL is below the diagnostic limit and reflects adequate glucose disposal. The 3-hour value helps assess sustained insulin action, but 130 mg/dL does not meet the criteria for GDM.
Take home points
- The 2-hour OGTT threshold for GDM diagnosis is ≥155 mg/dL.
- GDM diagnosis requires 2 or more values exceeding standard cutoffs.
- Fasting, 1-hour, and 3-hour thresholds are 95, 180, and 140 mg/dL respectively.
- OGTT reflects insulin resistance and placental hormone effects in pregnancy.
Which diagnostic criterion for the 75-gram 2-hour Oral Glucose Tolerance Test (OGTT) for GDM is considered abnormal if met or exceeded for the fasting glucose level?
Explanation
Gestational diabetes mellitus (GDM) is diagnosed using the 75-gram, 2-hour OGTT, which evaluates glucose tolerance, insulin resistance, placental hormone effects, and beta-cell function. The test is performed after an 8–14 hour fast and involves measuring plasma glucose at fasting, 1 hour, and 2 hours. Diagnostic thresholds are: fasting ≥92 mg/dL, 1-hour ≥180 mg/dL, and 2-hour ≥153 mg/dL. Meeting or exceeding any one of these values confirms GDM. The fasting value reflects hepatic glucose output and basal insulin activity.
Rationale for correct answer
2. A fasting glucose level of ≥92 mg/dL is the diagnostic threshold for GDM using the 75-gram OGTT. This value indicates impaired fasting glucose and reflects early beta-cell dysfunction. The question asks for the abnormal criterion, and 92 mg/dL is the correct cutoff for fasting glucose.
Rationale for incorrect answers
1. A fasting glucose of 80 mg/dL is within the normal range and does not meet the diagnostic threshold. It reflects adequate basal insulin function and hepatic glucose regulation. This value is not considered abnormal in the context of GDM screening.
3. A fasting glucose of 100 mg/dL exceeds the diagnostic threshold but is not the criterion used for GDM diagnosis. While it may indicate impaired fasting glucose in non-pregnant adults, the GDM threshold is lower due to pregnancy-induced insulin resistance. The correct cutoff is 92 mg/dL.
4. A fasting glucose of 126 mg/dL meets the criteria for overt diabetes, not GDM. This value suggests pre-existing diabetes mellitus and requires immediate management. GDM is diagnosed with lower thresholds specific to pregnancy physiology, and 126 mg/dL is beyond that scope.
Take home points
- Fasting glucose ≥92 mg/dL confirms GDM in the 75-gram OGTT.
- Only one abnormal value is needed for diagnosis.
- A fasting glucose ≥126 mg/dL indicates overt diabetes, not GDM.
- Pregnancy-specific thresholds are lower due to increased insulin resistance.
Which complication is associated with macrosomia in GDM?
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy. It results from insulin resistance, placental hormones, and maternal hyperglycemia, especially in the second and third trimesters. Elevated maternal glucose crosses the placenta, stimulating fetal insulin production, which acts as a growth hormone, leading to macrosomia. Macrosomia is defined as birth weight >4,000 g or >4,500 g depending on criteria. It increases risk for birth trauma, shoulder dystocia, and cesarean delivery. GDM also predisposes to neonatal hypoglycemia, polycythemia, and respiratory distress.
Rationale for correct answer
2. Shoulder dystocia occurs when the fetal anterior shoulder becomes impacted behind the maternal pubic symphysis during delivery. In macrosomic infants, excessive fetal size and fat deposition in the shoulders increase this risk. GDM-induced hyperinsulinemia promotes disproportionate growth of the shoulders relative to the head, making vaginal delivery difficult and increasing risk of brachial plexus injury.
Rationale for incorrect answers
1. Preterm labor is not directly caused by macrosomia. GDM is more commonly associated with post-term pregnancies due to delayed fetal lung maturity. While poorly controlled diabetes may increase risk of polyhydramnios, which can contribute to preterm labor, macrosomia itself is not a causative factor.
3. Low birth weight is the opposite of macrosomia. GDM leads to fetal overgrowth due to persistent maternal hyperglycemia and fetal hyperinsulinemia. Low birth weight (<2,500 g) is more typical in conditions like placental insufficiency, hypertension, or intrauterine growth restriction, not GDM.
4. Fetal anemia is not a complication of macrosomia. GDM does not impair fetal erythropoiesis or cause hemolysis. Anemia may result from fetomaternal hemorrhage, isoimmunization, or parvovirus B19, but not from the metabolic effects of GDM or macrosomia.
Take home points
- Macrosomia in GDM increases risk of shoulder dystocia due to fetal hyperinsulinemia.
- GDM causes fetal overgrowth, not low birth weight or anemia.
- Shoulder dystocia is a mechanical delivery complication linked to excessive fetal size.
Which of the following is a common fetal complication of uncontrolled Gestational Diabetes Mellitus related to high insulin levels interfering with lung maturation?
Explanation
Gestational diabetes mellitus (GDM) causes maternal hyperglycemia, which crosses the placenta and stimulates fetal insulin production. Fetal hyperinsulinemia interferes with surfactant synthesis, delaying lung maturation. Surfactant is critical for alveolar stability and gas exchange. Insulin antagonizes cortisol, which normally promotes surfactant production. This leads to increased risk of Respiratory Distress Syndrome (RDS), especially in infants born before 38 weeks. GDM also predisposes to macrosomia, hypoglycemia, and birth trauma, but RDS is specifically linked to delayed pulmonary development.
Rationale for correct answer
3. Respiratory Distress Syndrome (RDS) results from insufficient surfactant in immature lungs. In GDM, fetal hyperinsulinemia suppresses cortisol-induced surfactant synthesis, delaying lung maturation. This leads to alveolar collapse, hypoxia, and increased work of breathing. RDS is common in infants of diabetic mothers, even at term, due to biochemical immaturity.
Rationale for incorrect answers
1. Congenital heart defects are more common in pre-gestational diabetes, especially if poorly controlled during organogenesis (weeks 3–8). GDM typically develops later in pregnancy and does not affect early fetal cardiac development. Thus, heart defects are not a direct complication of GDM-related hyperinsulinemia.
2. Spina bifida is a neural tube defect linked to folate deficiency and teratogenic exposures during early pregnancy. It is not caused by fetal hyperinsulinemia or GDM. GDM develops after neural tube closure (by week 4), so it does not interfere with neural tube formation.
4. Cleft lip and palate result from failure of facial fusion during embryogenesis, typically between weeks 4 and 7. GDM arises later and does not affect craniofacial development. These defects are associated with genetic syndromes and maternal exposures, not insulin-mediated effects.
Take home points
- Fetal hyperinsulinemia in GDM delays surfactant production, increasing risk of RDS.
- RDS can occur even in term infants of diabetic mothers due to biochemical lung immaturity.
- Congenital anomalies like heart defects and spina bifida are linked to pregestational diabetes, not GDM.
- Cleft lip and palate are not complications of GDM; they occur earlier in embryogenesis.
What is the primary goal of medical nutrition therapy in gestational diabetes?
Explanation
Medical nutrition therapy in gestational diabetes mellitus (GDM) aims to regulate maternal glucose levels through dietary modifications. The goal is to achieve euglycemia, defined as fasting glucose <95 mg/dL, 1-hour postprandial <140 mg/dL, and 2-hour postprandial <120 mg/dL. Controlled glucose prevents macrosomia, neonatal hypoglycemia, and birth trauma. Nutrition plans balance complex carbohydrates, lean proteins, and healthy fats while avoiding simple sugars. Caloric intake is individualized based on BMI, gestational age, and fetal growth. Excessive restriction or overcompensation can worsen outcomes.
Rationale for correct answer
2. The goal of medical nutrition therapy in GDM is to achieve euglycemia and prevent maternal and fetal complications. This includes avoiding hyperglycemia-induced macrosomia, neonatal hypoglycemia, and preeclampsia. Balanced meals with controlled carbohydrate portions help maintain glucose within target ranges. Therapy is tailored to support fetal growth while minimizing risks.
Rationale for incorrect answers
1. Restricting all carbohydrate intake is not recommended. Carbohydrates are essential for fetal energy and maternal metabolism. The focus is on choosing complex carbohydrates with low glycemic index, not eliminating them. Total restriction can lead to ketosis, which is harmful to the fetus.
3. Rapid weight loss is contraindicated in pregnancy. Nutritional therapy aims for adequate weight gain based on pre-pregnancy BMI. Weight loss can impair fetal growth and increase risk of nutrient deficiencies. The goal is metabolic control, not weight reduction.
4. Increasing protein intake exclusively does not address glucose regulation. While protein supports fetal development, GDM management requires carbohydrate control and balanced macronutrients. Excess protein without carbohydrate moderation does not prevent hyperglycemia or its complications.
Take home points
- Medical nutrition therapy in GDM targets euglycemia to prevent fetal and maternal complications.
- Carbohydrates should be moderated, not eliminated, with focus on complex sources.
- Rapid weight loss is unsafe in pregnancy and not a therapeutic goal.
- Balanced macronutrient intake is essential; protein alone does not control glucose.
A pregnant client with GDM is taught to perform regular exercise. Which type of exercise is generally recommended for pregnant women with GDM?
Explanation
Gestational diabetes mellitus (GDM) is a glucose intolerance first recognized during pregnancy. It results from insulin resistance, placental hormones, and increased adiposity. GDM typically emerges in the second or third trimester due to rising levels of human placental lactogen, cortisol, and progesterone, which antagonize insulin. Fasting plasma glucose ≥92 mg/dL or 2-hour OGTT ≥153 mg/dL confirms diagnosis. Exercise improves glucose uptake, reduces insulin resistance, and lowers postprandial glucose levels. Safe physical activity enhances maternal cardiovascular health and reduces macrosomia risk without compromising fetal oxygenation.
Rationale for correct answer
2. Moderate-intensity activities like brisk walking or swimming are recommended because they enhance insulin sensitivity and reduce postprandial glucose without causing maternal hypoxia or uterine stress. These exercises maintain aerobic metabolism and avoid anaerobic thresholds, which could elevate lactate and catecholamines. Brisk walking at 3.5 mph or swimming for 30 minutes daily improves glycemic control and reduces fetal overgrowth risk.
Rationale for incorrect answers
1. High-intensity interval training is contraindicated in pregnancy with GDM due to risk of maternal hypoxia and fetal acidosis. These exercises exceed 85% of maximum heart rate, increasing catecholamine release and uterine blood flow redistribution. This can impair placental perfusion and elevate risk of preterm labor or fetal distress.
3. Weightlifting with heavy weights is discouraged due to increased intra-abdominal pressure and risk of valsalva-induced hypotension. Heavy resistance training may compromise venous return, reduce uteroplacental perfusion, and increase risk of musculoskeletal injury. It does not significantly improve insulin sensitivity compared to aerobic activity.
4. Contact sports pose risk of abdominal trauma and placental abruption. Activities like soccer or basketball involve rapid direction changes, falls, and collisions, which can lead to uterine injury, premature rupture of membranes, or fetal compromise. These are contraindicated throughout pregnancy regardless of GDM status.
Take home points
- Moderate-intensity aerobic exercise improves insulin sensitivity in GDM.
- High-intensity and contact sports increase risk of fetal compromise.
- Resistance training is not first-line for glycemic control in pregnancy.
Which of the following statements are accurate regarding the role of pregnancy hormones? Select all that apply
Explanation
Pregnancy hormones and glucose metabolism play a central role in maternal-fetal energy balance. During pregnancy, placental hormones, cortisol, progesterone, and human placental lactogen (hPL) progressively increase maternal insulin resistance, especially in the second and third trimesters. This adaptation ensures a steady glucose gradient favoring fetal uptake. hPL and cortisol antagonize insulin action, while estrogen and progesterone modulate vascular tone and uterine growth. The net effect is a diabetogenic state that prioritizes fetal nutrition. Fasting glucose may remain normal, but postprandial spikes are common due to reduced insulin efficacy.
Rationale for correct answers
2. Cortisol exhibits anti-insulin effects by promoting gluconeogenesis and reducing peripheral glucose uptake. It increases hepatic glucose output and impairs insulin receptor signaling, contributing to the insulin-resistant state of pregnancy.
3. Human placental lactogen (hPL) increases maternal insulin resistance by interfering with insulin receptor function and promoting lipolysis. This ensures maternal glucose is spared for fetal use and enhances free fatty acid availability for maternal metabolism.
5. These hormones ensure continuous glucose supply to the fetus by inducing maternal insulin resistance, thereby maintaining elevated maternal glucose levels. This facilitates passive glucose diffusion across the placenta to meet fetal metabolic demands.
Rationale for incorrect answers
1. Progesterone does not increase insulin sensitivity; instead, it contributes to insulin resistance by impairing insulin receptor signaling and promoting lipogenesis. Its vasodilatory effects may enhance uterine perfusion but do not improve glucose uptake.
4. Estrogen does not directly stimulate insulin production. While it may influence pancreatic β-cell mass and vascularization, its primary role is in modulating vascular tone and uterine growth. It does not act as a direct insulin secretagogue.
Take home points
- hPL and cortisol are key drivers of insulin resistance in pregnancy.
- Progesterone and estrogen modulate uterine and vascular physiology, not insulin sensitivity.
- Maternal insulin resistance ensures fetal glucose supply.
- Estrogen does not directly stimulate insulin secretion.
Which of the following are fetal complications of GDM? Select all that apply
Explanation
Gestational Diabetes Mellitus (GDM) is a condition characterized by glucose intolerance that develops during pregnancy due to placental hormone–induced insulin resistance. The resulting maternal hyperglycemia leads to fetal hyperinsulinemia, which significantly impacts fetal growth and organ maturity. These metabolic disturbances contribute to both macrosomia and organ immaturity, with a wide spectrum of fetal complications depending on the timing and control of maternal glucose levels.
Rationale for correct answers
1. Macrosomia occurs because excess maternal glucose crosses the placenta, stimulating fetal pancreatic β-cells to produce large amounts of insulin. Insulin acts as a potent growth-promoting hormone, leading to increased deposition of adipose tissue and glycogen, especially in the shoulders and trunk. This results in a birth weight >4,000 g and predisposes the neonate to shoulder dystocia and birth trauma.
2. Neonatal hypoglycemia develops after birth when the maternal glucose supply abruptly stops but the neonate’s high insulin production persists. The elevated insulin rapidly lowers neonatal blood glucose to <40 mg/dL. This can cause clinical signs such as jitteriness, lethargy, seizures, and apnea within the first few hours of life.
4. Respiratory distress syndrome (RDS) occurs because insulin inhibits surfactant synthesis in type II pneumocytes, delaying pulmonary maturity. Surfactant deficiency leads to alveolar collapse and hypoxemia after birth. Despite often being large for gestational age, infants of diabetic mothers have lungs functionally immature for their gestational age.
5. Fetal growth restriction (FGR) may occur in long-standing or poorly controlled vascular GDM, where placental insufficiency limits nutrient and oxygen delivery. Chronic fetal hypoxia results in symmetric or asymmetric growth restriction, depending on the timing of vascular compromise. This is more typical in mothers with preexisting diabetes complicated by microangiopathy.
Rationale for incorrect answers
3. Microsomia is not a feature of GDM. The predominant metabolic effect of maternal hyperglycemia is fetal overgrowth, not undergrowth, because of increased insulin-mediated anabolic activity. Microsomia generally occurs in cases of malnutrition, placental insufficiency, or chronic fetal hypoxia rather than maternal hyperglycemia.
Take home points
- Fetal exposure to maternal hyperglycemia causes hyperinsulinemia, leading to macrosomia and metabolic complications.
- Hypoglycemia develops in the neonate after birth due to persistent insulin secretion.
- RDS occurs from delayed surfactant synthesis caused by high fetal insulin levels.
- Growth restriction occurs in diabetic pregnancies complicated by vascular disease and placental insufficiency.
When teaching a client about self-monitoring of blood glucose for Gestational Diabetes Mellitus, which of the following statements indicates proper understanding? Select all that apply
Explanation
Gestational Diabetes Mellitus is a glucose intolerance first recognized during pregnancy, typically in the second or third trimester. It results from placental hormones, insulin resistance, and beta-cell dysfunction. Blood glucose targets are fasting <95 mg/dL, 1-hour postprandial <140 mg/dL, and 2-hour postprandial <120 mg/dL. Accurate self-monitoring, dietary control, and insulin therapy are essential to prevent fetal macrosomia, neonatal hypoglycemia, and maternal complications. Monitoring must be consistent, hygienic, and correlated with dietary intake to guide therapy adjustments.
Rationale for correct answers
2. Hand hygiene is essential to prevent contamination that may falsely elevate glucose readings. Residue from food or lotions can interfere with the accuracy of capillary blood glucose results. Washing with soap and water before testing ensures validity of the measurement.
3. Recording both glucose values and food intake allows for pattern recognition and dietary adjustments. This helps correlate glycemic excursions with specific meals and supports individualized nutritional counseling. It also aids providers in evaluating therapeutic effectiveness.
5. Testing fasting and 1–2 hours postprandial captures both baseline and post-meal glucose fluctuations. This timing reflects insulin response and helps detect postprandial hyperglycemia, which is a major contributor to fetal overgrowth. It aligns with clinical guidelines for gestational diabetes monitoring.
Rationale for incorrect answers
1. Checking only once daily in the morning misses postprandial spikes, which are critical in gestational diabetes. Fasting glucose alone does not reflect meal-induced hyperglycemia or overall glycemic control. This approach is insufficient for therapeutic decision-making and fetal risk reduction.
4. Expired test strips may yield inaccurate results due to degradation of enzyme reagents or altered electrochemical properties. Even if stored properly, expiration affects reliability. Using them compromises clinical decisions and may lead to inappropriate insulin dosing or dietary changes.
Take home points
- Gestational diabetes requires frequent, timed glucose monitoring to prevent fetal and maternal complications.
- Postprandial glucose levels are more predictive of fetal macrosomia than fasting levels alone.
- Accurate readings depend on proper technique, including hand hygiene and valid test strips.
- Recording food intake alongside glucose values supports individualized dietary management.
Which of the following are benefits of breastfeeding for women with GDM? Select all that apply
Explanation
Breastfeeding in Gestational Diabetes Mellitus offers maternal metabolic benefits and neonatal protection. It enhances insulin sensitivity, supports weight regulation, and reduces long-term risk of type 2 diabetes. Lactation increases energy expenditure, mobilizes fat stores, and improves glucose metabolism. It also lowers maternal blood pressure via oxytocin-mediated vasodilation. Breastfeeding does not increase fetal weight and has limited direct impact on neonatal jaundice, though early feeding promotes bilirubin clearance.
Rationale for correct answers
1. Breastfeeding improves insulin sensitivity by enhancing glucose uptake and reducing insulin resistance. Lactation mobilizes glucose for milk production, decreasing circulating glucose levels and improving metabolic control postpartum.
2. Lactation promotes weight loss through increased energy expenditure, averaging 500–700 kcal/day. Mobilization of adipose tissue and hormonal shifts support postpartum fat reduction, especially in abdominal stores.
5. Breastfeeding lowers maternal blood pressure via oxytocin release, which induces vasodilation and reduces sympathetic tone. This effect is sustained with regular nursing and contributes to cardiovascular protection.
Rationale for incorrect answers
3. Breastfeeding does not increase fetal weight. Fetal weight is determined by intrauterine factors such as maternal glucose levels and placental function. Postnatal feeding influences neonatal growth, not fetal development. Breastfeeding may actually reduce risk of childhood obesity.
4. While early breastfeeding helps stimulate meconium passage, which aids bilirubin excretion, it does not directly reduce neonatal jaundice. Inadequate intake or delayed lactogenesis can worsen jaundice. Phototherapy remains the primary treatment for hyperbilirubinemia.
Take home points
- Breastfeeding improves insulin sensitivity and reduces risk of type 2 diabetes in women with GDM.
- Lactation promotes postpartum weight loss through increased energy demands.
- Oxytocin release during breastfeeding lowers maternal blood pressure.
- Breastfeeding does not affect fetal weight and has limited impact on neonatal jaundice.
Which of the following are key recommendations for postpartum care and long-term prevention for a woman with a history of Gestational Diabetes Mellitus? Select all that apply
Explanation
Postpartum care in Gestational Diabetes Mellitus focuses on preventing progression to type 2 diabetes, optimizing metabolic health, and supporting cardiovascular protection. Women with prior GDM have a 7-fold increased risk of developing type 2 diabetes. Annual screening, lifestyle modification, and weight control are essential. Breastfeeding improves insulin sensitivity and should be encouraged. Glucose monitoring continues beyond the postpartum screen, especially in high-risk individuals. Early intervention reduces long-term complications.
Rationale for correct answers
1. Lifelong annual screening is recommended due to persistent insulin resistance and increased risk of type 2 diabetes. Even if postpartum glucose normalizes, metabolic dysfunction may progress silently. Annual oral glucose tolerance tests or HbA1c help detect early changes.
3. Regular physical activity improves glucose metabolism and enhances insulin sensitivity. It reduces visceral fat, lowers cardiovascular risk, and supports weight maintenance. Activities like brisk walking, swimming, or resistance training are effective.
4. Maintaining a healthy weight reduces adipose-driven insulin resistance and lowers risk of metabolic syndrome. BMI <25 kg/m² is ideal. Weight control also improves lipid profile and blood pressure, reducing long-term cardiovascular burden.
Rationale for incorrect answers
2. Avoiding breastfeeding is not recommended. Breastfeeding improves glucose regulation and enhances insulin sensitivity. It lowers maternal risk of type 2 diabetes and supports neonatal health. Glucose fluctuations during lactation are minimal and physiologically beneficial.
5. No further glucose monitoring is incorrect. A normal postpartum screen does not eliminate future risk. Beta-cell dysfunction may persist, and insulin resistance can worsen over time. Continued monitoring is essential for early detection and prevention.
Take home points
- Women with prior GDM require lifelong annual diabetes screening due to high conversion risk.
- Regular physical activity and weight control are critical for metabolic health.
- Breastfeeding improves insulin sensitivity and should be encouraged.
- Postpartum glucose normalization does not eliminate future diabetes risk.
Exams on Diabetes Mellitus
Custom Exams
Login to Create a Quiz
Click here to loginLessons
 Naxlex
Just Now
Naxlex
Just Now
- Objectives
- Introduction
- Pathophysiology Of Diabetes In Pregnancy
- Practice Exercise 1
- Types Of Diabetes In Pregnancy
- Risk Factors For Gestational Diabetes Mellitus
- Practice Exercise 2
- Screening And Diagnosis Of Gestational Diabetes Mellitus
- Practice Exercise 3
- Maternal And Fetal Complications Of Diabetes In Pregnancy
- Practice Exercise 4
- Management Of Diabetes In Pregnancy
- Practice Exercise 5
- Fetal Surveillance And Monitoring In Gdm
- Practice Exercise 6
- Patient Education And Health Promotion
- Practice Exercise 7
- Summary
- Comprehensive Questions
Notes Highlighting is available once you sign in. Login Here.
Objectives
At the end of this topic, the learner should be able to:
- Explain the pathophysiology of diabetes in pregnancy, emphasizing alterations in glucose metabolism, hormonal influences, maternal adaptations, and fetal glucose regulation.
- Differentiate between preexisting diabetes mellitus (Type 1 and Type 2) and gestational diabetes mellitus (GDM) based on etiology, timing of onset, and pathophysiological mechanisms.
- Identify maternal and fetal risk factors that predispose to the development of gestational diabetes mellitus, distinguishing modifiable from non-modifiable elements.
- Discuss the screening and diagnostic procedures for gestational diabetes mellitus, including one-step and two-step testing, glucose challenge test (GCT), and oral glucose tolerance test (OGTT), and interpret diagnostic thresholds.
- Describe the maternal complications of diabetes in pregnancy such as diabetic ketoacidosis (DKA), preeclampsia, polyhydramnios, and infections, including their pathogenesis and clinical implications.
- Analyze the fetal complications associated with maternal diabetes, including congenital anomalies, macrosomia, intrauterine growth restriction (IUGR), respiratory distress syndrome, and neonatal hypoglycemia.
- Outline evidence-based management strategies for diabetes in pregnancy, including preconception counseling, medical nutrition therapy (MNT), physical activity, pharmacological interventions (insulin and oral hypoglycemics), and glucose monitoring.
- Explain nursing management during labor and delivery, focusing on glycemic control, fetal monitoring, and prevention of maternal and neonatal complications.
- Discuss postpartum management, emphasizing glucose regulation, maternal recovery, and strategies to prevent long-term metabolic disease.
- Evaluate fetal surveillance techniques such as nonstress test (NST), biophysical profile (BPP), amniotic fluid index (AFI), and ultrasound for growth monitoring, and interpret findings in the context of maternal diabetes.
- Implement comprehensive patient education on self-monitoring of blood glucose, recognition and management of hypoglycemia and hyperglycemia, and the importance of adherence to prescribed therapy.
- Promote breastfeeding and long-term lifestyle modifications that reduce the risk of progression from gestational diabetes to Type 2 diabetes mellitus.
- Apply critical thinking in answering NCLEX-style practice questions on the diagnosis, management, and nursing care of patients with diabetes in pregnancy.
Introduction
Diabetes mellitus in pregnancy represents a spectrum of metabolic disturbances that significantly influence both maternal and fetal outcomes. It is characterized by varying degrees of carbohydrate intolerance resulting in hyperglycemia, which may be preexisting (Type 1 or Type 2 diabetes mellitus) or first recognized during pregnancy (gestational diabetes mellitus).
Pregnancy induces profound hormonal, metabolic, and vascular changes designed to meet fetal nutritional demands. These adaptations include increased insulin resistance, enhanced lipolysis, and altered glucose utilization. While these changes are physiological, excessive insulin resistance or inadequate pancreatic β-cell compensation leads to maternal hyperglycemia.
Hyperglycemia during pregnancy poses risks for maternal complications such as preeclampsia, polyhydramnios, and diabetic ketoacidosis, and for fetal complications including congenital malformations, macrosomia, and neonatal hypoglycemia. Furthermore, gestational diabetes increases the mother’s lifetime risk of developing Type 2 diabetes and predisposes the child to obesity and metabolic syndrome later in life.
Early identification, tight glycemic control, and multidisciplinary management are critical to optimizing outcomes. The nurse plays a pivotal role in patient education, glucose monitoring, nutritional guidance, and emotional support—ensuring safe pregnancy progression and delivery for both mother and infant.
Pathophysiology Of Diabetes In Pregnancy
1.1 Overview Of Glucose Metabolism In Pregnancy
- Pregnancy induces dynamic changes in maternal carbohydrate metabolism that occur in a predictable pattern across trimesters, designed to optimize substrate supply to the fetus while maintaining maternal homeostasis.
- In early pregnancy (first trimester) maternal metabolism is relatively anabolic: increased maternal insulin sensitivity and enhanced glycogen and fat storage occur to build maternal energy reserves. These changes facilitate implantation and organogenesis and provide substrates for later fetal growth.
- In late pregnancy (second and third trimesters) maternal metabolism shifts to a catabolic state characterized by progressive insulin resistance, increased lipolysis, and mobilization of maternal fat stores to provide continuous glucose, free fatty acids, and ketone bodies for the fetus.
- Glucose crosses the placenta by facilitated diffusion via GLUT transporters (primarily GLUT1). Fetal glucose concentration is normally 70–80% of maternal glucose; therefore, maternal hyperglycemia leads directly to fetal hyperglycemia.
- Fetal insulin does not cross the placenta; fetal pancreatic β-cells respond to increased fetal glucose by hypertrophy and hyperplasia and by secreting increased insulin, which acts as a fetal growth factor and promotes adiposity.
- The balance between maternal insulin secretion and insulin resistance determines maternal glycemia. If β-cell compensation is adequate, normoglycemia is maintained; if compensation is inadequate, maternal hyperglycemia develops, resulting in gestational diabetes or exacerbation of preexisting diabetes.
Nursing Insights
- Nurses must understand that pregnancy physiology intentionally reduces maternal insulin sensitivity in late gestation to prioritize fetal nutrient supply; therefore, deterioration of glycemic control commonly occurs in the second and third trimesters. Practical implication: anticipate increased insulin requirements during late pregnancy and adjust therapy based on frequent glucose monitoring.
- Recognize GLUT1-mediated placental glucose transfer implies that any maternal hyperglycemia is transmitted to the fetus quickly; therefore, transient maternal hyperglycemia (postprandial peaks) can have immediate fetal metabolic consequences.
- When caring for pregnant patients, chart trends rather than single values because physiologic gestational changes alter expected glucose patterns across trimesters.
1.2 Hormonal Influences On Insulin Resistance
- Multiple placental and maternal hormones produce progressive insulin antagonism during pregnancy. The net effect is decreased maternal insulin sensitivity and increased hepatic glucose production. Key mediators include:
- Human placental lactogen (hPL) (also called human chorionic somatomammotropin): produced by syncytiotrophoblasts; it increases maternal lipolysis and decreases maternal insulin sensitivity, thereby increasing maternal free fatty acids and glucose availability for the fetus.
- Placental growth hormone (PGH): gradually replaces pituitary growth hormone in late pregnancy and increases maternal insulin resistance and hepatic gluconeogenesis.
- Progesterone and estrogen: exert complex modulatory effects that contribute to insulin resistance through alterations in insulin receptor signaling and adipose tissue metabolism.
- Cortisol: maternal adrenal cortisol rises in pregnancy; cortisol exerts anti-insulin effects by promoting gluconeogenesis and decreasing peripheral glucose uptake.
- Prolactin: influences β-cell mass and insulin secretion; has adaptive roles but can contribute to altered glucose homeostasis.
- Catecholamines: elevated in stress and can transiently raise glucose through glycogenolysis and gluconeogenesis.
- Mechanisms of insulin resistance:
- Post-receptor signaling defects: decreased insulin-stimulated glucose transporter translocation (reduced GLUT4 activity in maternal skeletal muscle and adipose tissue).
- Increased adipokines and inflammatory mediators from adipose tissue that impair insulin receptor signaling (e.g., TNF-α, IL-6).
- Increased hepatic glucose output due to hormonal stimulation of gluconeogenic enzymes (PEPCK, G6Pase).
Table — Hormones and Their Major Metabolic Effects in Pregnancy
|
Hormone |
Source |
Principal Metabolic Effects |
|
Human placental lactogen (hPL) |
Placenta |
↑ Lipolysis; ↑ Insulin resistance; fetal nutrient supply |
|
Placental growth hormone (PGH) |
Placenta |
↑ Hepatic gluconeogenesis; ↑ Insulin resistance |
|
Progesterone |
Ovary + Placenta |
Modulates glucose uptake; contributes to insulin resistance |
|
Estrogen |
Ovary + Placenta |
Alters insulin receptor signaling; affects adiposity |
|
Cortisol |
Maternal adrenal |
↑ Gluconeogenesis; ↓ peripheral glucose uptake |
|
Prolactin |
Pituitary |
↑ β-cell mass/function (adaptive) |
Nursing Insights
- Human placental lactogen is a dominant mediator of pregnancy-associated insulin resistance; when asked in exams, identify hPL as the hormone most directly correlated with maternal insulin resistance and increased free fatty acids. Clinically, anticipate insulin requirements to increase as hPL levels rise (mid-to-late pregnancy).
- Understand that multiple hormones cumulatively produce insulin resistance; therefore, single-hormone-focused interventions are insufficient — management must be multifactorial (nutrition, exercise, pharmacotherapy).
- For patients with intercurrent illness or corticosteroid therapy, expect acute worsening of hyperglycemia due to additive cortisol effects — increase monitoring and adjust therapy promptly.
1.3 Differences Between Preexisting Diabetes Mellitus And Gestational Diabetes Mellitus
- Timing and diagnosis:
- Preexisting diabetes mellitus is present before conception and includes Type 1 (autoimmune β-cell destruction with absolute insulin deficiency) and Type 2 (insulin resistance with relative insulin deficiency) diabetes. These patients carry risks from the earliest stages of embryogenesis, including congenital anomalies if glycemic control is poor during organogenesis (first trimester).
- Gestational diabetes mellitus (GDM) is glucose intolerance first recognized in pregnancy, usually diagnosed in the second or third trimester. GDM generally does not cause congenital anomalies attributable to hyperglycemia in the first trimester unless undiagnosed preexisting diabetes existed.
- Pathophysiologic substrate:
- Type 1: autoimmune-mediated β-cell loss → absolute insulin deficiency.
- Type 2: chronic insulin resistance often with β-cell dysfunction; obesity common.
- GDM: relative β-cell insufficiency that cannot compensate for pregnancy-induced insulin resistance.
- Maternal risks:
- Preexisting diabetes: higher rates of congenital malformations, miscarriage, exacerbation of microvascular disease (retinopathy, nephropathy), and chronic diabetes complications that can progress during pregnancy.
- GDM: higher risk of hypertensive disorders, cesarean delivery, and progression to Type 2 diabetes postpartum.
- Fetal/neonatal risks:
- Both conditions increase risks of macrosomia, birth trauma, neonatal hypoglycemia, and respiratory morbidity; preexisting diabetes carries a greater risk of structural congenital anomalies (cardiac, neural tube) due to hyperglycemia during organogenesis.

- Management differences:
- Preexisting diabetes requires preconception optimization of glycemic control (A1c target individualized, often <7% or lower if safe), adjustment of medications preconception (e.g., insulin preferred, teratogenic drugs stopped), and early pregnancy high-intensity surveillance.
- GDM management typically begins with medical nutrition therapy and glucose monitoring; pharmacologic therapy is initiated if glycemic targets are not met. Screening at 24–28 weeks is standard for average-risk women; high-risk women are screened earlier.
Nursing Insights
- For examinations and clinical practice, remember: congenital anomalies are primarily a risk of preexisting diabetes due to hyperglycemia during organogenesis; in contrast, GDM typically manifests after organogenesis and therefore is less associated with structural malformations.
- Clinically, a woman presenting with hyperglycemia at first prenatal visit should be evaluated for overt diabetes (preexisting) rather than assumed to have GDM; management and counseling differ substantively.
- Nurses should screen prior diagnosis and medication lists at the initial visit: if the patient uses oral hypoglycemics or has an A1c consistent with diabetes, manage as preexisting diabetes and initiate multidisciplinary care immediately.
1.4 Maternal Metabolic Adaptations To Pregnancy
- Pregnancy features both anabolic and catabolic phases with biochemical adaptations:
- Anabolic phase (first half of pregnancy): increased insulin sensitivity, increased maternal adipose deposition, increased hepatic glycogen stores, and increased appetite. These changes conserve energy and build maternal nutrient stores.
- Catabolic phase (second half of pregnancy): progressive insulin resistance, increased mobilization of lipids (elevated maternal free fatty acids and glycerol), enhanced hepatic gluconeogenesis, and increased basal metabolic rate. These changes ensure continuous nutrient flow to the fetus.
- Alterations in maternal lipid metabolism: pregnancy induces hyperlipidemia (↑ triglycerides, ↑ LDL cholesterol), which supplies substrates for placental steroidogenesis and fetal fat accretion. Elevated maternal lipids can also worsen insulin resistance.
- Protein metabolism: maternal protein turnover increases to supply fetal growth demands; maternal nitrogen balance is maintained by increased dietary intake and enhanced renal conservation.
- Energy balance and insulin requirements: insulin requirements typically decline slightly in early pregnancy, then rise progressively after 20 weeks and may double or triple by late third trimester in women with diabetes. Immediately postpartum, insulin sensitivity rapidly increases and insulin requirements drop precipitously; failure to anticipate this can cause maternal hypoglycemia.
Table — Summary of Maternal Metabolic Changes Across Pregnancy
|
Metabolic Domain |
Early Pregnancy (Anabolic) |
Late Pregnancy (Catabolic) |
|
Insulin sensitivity |
↑ |
↓ (progressive insulin resistance) |
|
Hepatic gluconeogenesis |
Baseline |
↑ |
|
Lipolysis |
Baseline |
↑ (↑ free fatty acids) |
|
Insulin requirements (diabetic patients) |
↓/stable |
↑ (may double/triple) |
|
Fetal glucose transfer |
Increasing |
Maximal (via GLUT1) |
Nursing Insights
- Anticipate increasing insulin requirements in the second and third trimesters for patients on insulin; schedule more frequent glucose checks and adjust sliding scales accordingly. Practical tip: document trends to guide dose titration rather than react to single abnormal readings.
- After delivery, anticipate rapid decrease in insulin needs; implement protocols for postpartum insulin dose reduction and vigilant hypoglycemia monitoring in the immediate postpartum period.
- Understand the metabolic rationale for counseling about diet composition: distributing carbohydrate intake and providing adequate protein and complex carbohydrates maintains maternal-fetal glucose homeostasis while reducing postprandial peaks.
1.5 Fetal Glucose Metabolism And Insulin Response
- The fetus relies predominantly on maternal glucose delivered via placental transfer; fetal endogenous gluconeogenesis is minimal until late gestation. Fetal glucose concentration is proportional to maternal glucose concentration, typically ~70–80% of maternal levels.
- Fetal pancreatic β-cells respond to increased glucose with increased insulin secretion. Fetal hyperinsulinemia is a key mediator of many diabetic pregnancy complications:
- Fetal macrosomia results from insulin-stimulated adipogenesis and somatic growth; fetal insulin acts as an anabolic growth factor.

- Polyhydramnios can occur due to fetal hyperglycemia-induced osmotic diuresis increasing fetal urine output.
- Delayed pulmonary maturation: fetal hyperinsulinemia antagonizes cortisol-induced surfactant synthesis, increasing the risk of respiratory distress syndrome (RDS) even at term or near-term.
- After birth, the abrupt interruption of maternal glucose supply combined with persistent neonatal hyperinsulinemia predisposes the neonate to hypoglycemia within the first hours after delivery. Risk is highest in neonates of mothers with poorly controlled diabetes or those with marked maternal hyperglycemia.
- Other fetal metabolic consequences: intermittent fetal hyperglycemia can increase fetal oxygen consumption and result in relative fetal hypoxemia, promoting polycythemia and hyperbilirubinemia after birth due to increased erythropoiesis and subsequent hemolysis.
Nursing Insights
- Recognize that fetal hyperinsulinemia is central to the pathogenesis of macrosomia, polyhydramnios, RDS, neonatal hypoglycemia, and polycythemia; thus, maternal glycemic control during pregnancy is the primary preventive strategy.
- Intrapartum nursing care should anticipate that neonates of diabetic mothers require early feeding and glucose monitoring within the first 1–2 hours after birth; arrange neonatal glucose checks per protocol (e.g., at 30–60 minutes, and serially thereafter) to detect hypoglycemia early.
- Understand the physiologic basis for increased risk of RDS in infants of diabetic mothers: counsel that even term infants may need respiratory support and monitoring, and coordinate neonatal respiratory preparedness during delivery.
Types Of Diabetes In Pregnancy
1.1 Preexisting Diabetes Mellitus (Type 1 And Type 2)
- Definition and general considerations
- Preexisting diabetes mellitus refers to hyperglycemic disorders that are present prior to conception and therefore expose the embryo and fetus to abnormal metabolic milieu from conception onward.
- Two principal types are Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM); each has distinct pathophysiology, clinical course, and obstetric implications that influence prenatal counseling, antenatal surveillance, intrapartum management, and postpartum follow-up.
- Type 1 Diabetes Mellitus (T1DM)
- Pathophysiology: autoimmune-mediated destruction of pancreatic β-cells leading to absolute insulin deficiency and requirement for exogenous insulin replacement.
- Clinical phenotype: often presents in childhood/adolescence but can present at any age; typically lean body habitus but not universally.
- Pregnancy implications:
- High risk for congenital anomalies if glycemic control is poor during organogenesis (first trimester).
- Increased risk of diabetic microvascular complications (retinopathy, nephropathy) progression during pregnancy; pregnancy may transiently worsen retinopathy.
- Greater variability in glycemic control with risk of hypoglycemia (especially in early pregnancy) due to increased insulin sensitivity in the first trimester and changing insulin requirements.
- Preconception optimization of glycemic control (A1c target individualized; frequently ≤7% if achievable without significant hypoglycemia) is crucial to reduce malformation risk.
- Type 2 Diabetes Mellitus (T2DM)
- Pathophysiology: chronic peripheral insulin resistance combined with relative β-cell dysfunction; frequently associated with obesity, dyslipidemia, hypertension, and metabolic syndrome.
- Clinical phenotype: commonly diagnosed in adulthood; often associated with overweight/obesity and features of insulin resistance.
- Pregnancy implications:
- Many women with T2DM are diagnosed before pregnancy but some have undiagnosed diabetes discovered at first prenatal visit.
- Risk for congenital anomalies exists if hyperglycemia is present during organogenesis; the degree of risk correlates with the level and duration of hyperglycemia.
- These women frequently have comorbidities (e.g., hypertension, dyslipidemia) that increase obstetric risk (preeclampsia, cesarean delivery).
- Management often requires transition from oral hypoglycemics to insulin preconception or in early pregnancy because many oral agents are not recommended in pregnancy (exceptions and evolving evidence exist for metformin in selected settings).
- Shared features and practical concerns for preexisting diabetes
- Both types require individualized preconception counseling, medication review, and glycemic optimization before conception.
- Women with preexisting diabetes need early fetal anatomic ultrasound, targeted screening for microvascular complications, and more intensive fetal surveillance in pregnancy.
- Insulin regimens almost always need adjustment during pregnancy; physiologic insulin needs fall in early pregnancy and then rise markedly in the second and third trimesters.
Nursing Insights
- Assess medication lists at the first prenatal visit: if the patient is on oral hypoglycemics, treat as potential T2DM and coordinate prompt evaluation of A1c and fasting glucose; do not assume GDM.
- Teach patients about the importance of preconception glycemic control; specifically document their last A1c and counsel on target values and risks of poor control in early pregnancy.
- Monitor for and document baseline microvascular disease (retinopathy, nephropathy): arrange ophthalmology review and renal function assessment early in pregnancy.
- Practical tip: instruct patients on how to adjust insulin with symptomatic hypoglycemia in early pregnancy and caution about nocturnal hypoglycemia; promote frequent SMBG (self-monitoring blood glucose) during periods of regimen change.
1.2 Gestational Diabetes Mellitus (GDM)
- Definition
- Gestational diabetes mellitus is glucose intolerance with onset or first recognition during pregnancy. It typically manifests in the second or third trimester when the physiological insulin resistance of pregnancy peaks. GDM usually resolves after delivery but indicates future risk for Type 2 diabetes.
- Pathophysiology (concise recap relevant here)
- Pregnancy-associated hormonal changes (hPL, placental growth hormone, cortisol, progesterone, estrogen) increase insulin resistance. GDM occurs when pancreatic β-cell insulin secretion is insufficient to overcome this resistance. The result is maternal hyperglycemia with maternal–fetal metabolic consequences.
- Clinical features and course
- GDM often is asymptomatic and detected by screening at 24–28 weeks in average-risk women or earlier in those at high risk.
- Glycemic abnormalities are most frequently postprandial hyperglycemia; fasting hyperglycemia is less commonly predominant but can occur.
- GDM is associated with excess fetal growth (macrosomia), polyhydramnios, birth trauma, neonatal hypoglycemia, and increased cesarean rates.
- Management overview (brief here; detailed management is in Section 12)
- Initial management focuses on medical nutrition therapy (MNT), physical activity, and SMBG. If glycemic targets are not achieved, pharmacologic therapy (insulin preferred; oral agents like metformin or glyburide used selectively) is initiated.
- Postpartum follow-up with a 75-g OGTT at 6–12 weeks postpartum is recommended to identify persistent diabetes.
Nursing Insights
- Do not delay screening in high-risk women—they may have overt preexisting diabetes rather than GDM; early screening at the first prenatal visit is important.
- Educate patients that GDM is a marker of future cardiometabolic risk; emphasize lifestyle modification postpartum to reduce risk of Type 2 diabetes.
- Practical patient education point: explain that successful initial management is often diet and exercise; clarify SMBG targets and frequency to empower self-management.
1.3 Classification Of Diabetes In Pregnancy
- Simplified classification schema (useful for clinical communication and documentation):
- Overt diabetes in pregnancy (preexisting diabetes)
- Type 1 diabetes mellitus
- Type 2 diabetes mellitus
- Previously undiagnosed diabetes identified in early pregnancy (A1c ≥6.5% or fasting glucose ≥126 mg/dL) — treat as overt diabetes.
- Gestational diabetes mellitus (GDM)
- GDM diagnosed in pregnancy (usually per 75-g one-step or 2-step approach).
- Subcategories for clinical nuance
- Diet-controlled GDM (managed with MNT alone)
- Pharmacologically treated GDM (requires insulin and/or oral agents)
- GDM with complications (e.g., polyhydramnios, macrosomia)
- Overt diabetes in pregnancy (preexisting diabetes)
- Clinical documentation and coding implications
- Distinguish in notes between pregestational (preexisting) and gestational diabetes for appropriate antenatal surveillance and for postpartum follow-up planning.
- Use objective criteria (A1c, fasting glucose, OGTT results) to classify and to guide management pathways.
Table — Key Clinical Differences at a Glance
|
Feature |
Preexisting Diabetes (T1/T2) |
GDM |
|
Onset |
Before conception or identified at first visit |
Typically diagnosed after 24 weeks |
|
Risk of congenital anomalies |
High if poor control during organogenesis |
Low unless undiagnosed preexisting diabetes |
|
Primary pathophysiology |
T1: absolute insulin deficiency; T2: chronic insulin resistance |
Relative β-cell insufficiency vs pregnancy insulin resistance |
|
Surveillance intensity |
High (early fetal ultrasound, microvascular monitoring) |
Moderate to high (growth scans, glycemic monitoring) |
|
Postpartum diabetes risk |
Often persists (T1/T2) |
Increased lifetime risk of T2DM; often resolves postpartum |
Nursing Insights
- When triaging pregnant patients, always determine whether diabetes is preexisting or gestational because immediate management, preconception counseling history, and surveillance differ markedly.
- Document key objective values (A1c, fasting glucose, OGTT results) in the chart so that any clinician can rapidly determine classification and next steps.
Practical bedside reminder: if A1c ≥6.5% or fasting glucose ≥126 mg/dL early in pregnancy, treat as overt diabetes and escalate referrals.
Risk Factors For Gestational Diabetes Mellitus
1.1 Modifiable Risk Factors
- Obesity and elevated pre-pregnancy BMI
- Pre-pregnancy BMI ≥30 kg/m² is a strong predictor of GDM due to preexisting insulin resistance mediated by adiposity and associated inflammatory adipokines (e.g., TNF-α, IL-6).
- Weight reduction prior to conception reduces GDM risk and improves pregnancy outcomes.
- Excessive gestational weight gain
- Weight gain above Institute of Medicine (IOM) recommendations increases insulin resistance and GDM risk; counseling on appropriate gestational weight gain tailored to pre-pregnancy BMI is essential.
- Poor diet quality
- Diets high in refined carbohydrates and saturated fats and low in fiber increase insulin resistance and glucose excursions. Dietary modification emphasizing complex carbohydrates, fiber, and balanced macronutrient distribution mitigates risk.
- Physical inactivity / Sedentary lifestyle
- Lack of regular moderate-intensity exercise reduces insulin-stimulated glucose uptake by skeletal muscle and increases GDM risk.
- Smoking
- Cigarette smoking has been associated with insulin resistance and adverse metabolic effects, contributing to higher GDM risk.
- Polycystic ovary syndrome (PCOS) (modifiable to an extent)
- PCOS is associated with insulin resistance; weight management and preconception optimization can reduce GDM risk.
- Previous GDM (modifiable risk via postpartum lifestyle)
- Women with prior GDM have a high recurrence risk; postpartum lifestyle interventions (diet, exercise, weight control) reduce recurrence.
Nursing Insights
- Implement targeted preconception counseling focusing on weight optimization and smoking cessation for women planning pregnancy; document BMI and provide specific, evidence-based weight goals.
- Provide concrete dietary guidance: recommend distribution of carbohydrates across meals and 2–3 snacks, prioritize complex carbohydrates and vegetable fiber, and avoid sugar-sweetened beverages.
- Encourage and prescribe (documented) moderate-intensity exercise (e.g., 30 minutes brisk walking most days) unless contraindicated; explain mechanisms (improved insulin sensitivity) to enhance adherence.
1.2 Non-Modifiable Risk Factors
- Advanced maternal age
- Maternal age ≥35 years is associated with increased risk of GDM, likely due to age-related decline in β-cell function and increased insulin resistance.
- Ethnicity/race
- Certain ethnic groups have higher GDM prevalence (e.g., Hispanic, South Asian, Native American, African descent). Ethnic predisposition likely reflects genetic predisposition and sociocultural determinants of metabolic risk.
- Family history of Type 2 diabetes (first-degree relative)
- A positive family history indicates genetic susceptibility to insulin resistance and β-cell dysfunction.
- History of macrosomic infant or prior obstetric history
- Previous delivery of a macrosomic infant (e.g., birth weight >4,000 g or large-for-gestational-age) or prior GDM increases recurrence risk.
- Prior impaired glucose tolerance or impaired fasting glucose
- Documented prediabetes prior to pregnancy predicts higher GDM risk.
- Genetic predisposition
- Polymorphisms related to insulin secretion and action increase risk; while not modifiable, they inform individualized risk assessment.
Table — Major Modifiable vs Non-Modifiable Risk Factors
|
Modifiable Risk Factors |
Non-Modifiable Risk Factors |
|
Obesity (pre-pregnancy BMI ≥30 kg/m²) |
Advanced maternal age (↑ risk with age) |
|
Excessive gestational weight gain |
Ethnic background (e.g., Hispanic, South Asian) |
|
Sedentary lifestyle |
Family history of T2DM |
|
Poor diet (high refined carbs, low fiber) |
Prior GDM or macrosomic infant |
|
Smoking |
Prior impaired glucose tolerance/prediabetes |
|
PCOS (partially modifiable via weight loss) |
Genetic predisposition |
Nursing Insights
- At booking/first prenatal visit, perform targeted risk stratification: record pre-pregnancy BMI, family history, ethnicity, obstetric history (previous macrosomia/GDM), and PCOS/other endocrine disorders. Use this stratification to determine timing of screening (early vs routine 24–28 weeks).
- Provide culturally sensitive education: tailor dietary and exercise recommendations to the patient's cultural food practices, socioeconomic context, and access to resources.
- Create a documented individualized care plan that lists modifiable risks with explicit, measurable interventions (e.g., "Patient will perform 30 minutes brisk walking 5 days/week" and "Patient will reduce sugar-sweetened beverage intake to 0/week").
Screening And Diagnosis Of Gestational Diabetes Mellitus
Screening and diagnosis of gestational diabetes mellitus (GDM) require clear understanding of the available approaches, standardized test procedures, diagnostic thresholds, and postpartum surveillance. The clinician must balance sensitivity and specificity of screening strategies with local practice patterns and patient risk profile. Below are comprehensive, detailed, and clinically practical notes that synthesize current, commonly used diagnostic approaches and the nursing implications for each step.
1.1 Screening Guidelines (One-Step And Two-Step Approaches)
- General principles:
- Screening for GDM identifies women with glucose intolerance that developed or was first recognized during pregnancy and allows timely interventions to reduce maternal and fetal complications.
- There are two widely used screening strategies: the one-step approach (IADPSG / WHO style using 75-g OGTT) and the two-step approach (ACOG/older U.S. practice using a non-fasting 50-g glucose challenge test followed by a diagnostic 100-g OGTT if the screen is positive). Choice of approach depends on institutional policy, population characteristics, and resource availability.
- One-Step Approach (75-g OGTT — IADPSG / WHO style):
- Single fasting 75-g oral glucose tolerance test performed at 24–28 weeks for average-risk women (earlier if high risk).
- Advantages: single diagnostic test; harmonizes screening/diagnosis; based on pregnancy outcomes (HAPO study) to identify women at increased risk of adverse outcomes.
- Disadvantages: increases the number of women diagnosed with GDM compared with two-step; requires fasting and more laboratory resources.
- Two-Step Approach (50-g GCT screening followed by 100-g OGTT diagnostic test):
- Step 1: Non-fasting 50-g glucose challenge test (GCT) at 24–28 weeks. If result ≥ threshold (commonly 130–140 mg/dL depending on institutional cutpoint), proceed to Step 2.
- Step 2: Fasting 100-g 3-hour OGTT with measurement of fasting, 1-hour, 2-hour, and 3-hour values; diagnostic thresholds (Carpenter-Coustan or NDDG) are applied to determine GDM.
- Advantages: fewer women undergo the full OGTT; more widely used historically in the U.S.; convenient initial non-fasting screen.
- Disadvantages: requires two clinical visits if positive screen; possible false negatives/positives depending on screening threshold.
Table — One-Step vs Two-Step Approach (Key Operational Differences)
|
Feature |
One-Step (75-g OGTT) |
Two-Step (50-g GCT → 100-g OGTT) |
|
Test timing |
24–28 weeks (earlier if high risk) |
24–28 weeks (earlier if high risk) |
|
Fasting required |
Yes |
No for GCT; Yes for OGTT |
|
Number of blood draws |
Fasting, 1h, 2h |
Non-fasting 1h (GCT); if positive → fasting, 1h, 2h, 3h (OGTT) |
|
Diagnostic thresholds |
IADPSG/WHO criteria |
Carpenter-Coustan or NDDG thresholds |
|
Pros |
Single diagnostic test; outcome-based |
Fewer full OGTTs; long historical use |
|
Cons |
More diagnoses; requires fasting |
Two steps; potential for extra visits |
Nursing Insights
- Know your institution’s protocol: before ordering or scheduling, verify whether the clinic/hospital uses one-step or two-step screening because patient preparation and scheduling differ.
- Educate patients clearly about fasting requirements and dietary preparation to avoid invalid results; failure to follow preparation increases false negatives/positives.
- For high-risk women (e.g., prior GDM, obesity, strong family history), the nurse should ensure early screening at the first prenatal visit in addition to the routine 24–28 weeks screen.
1.2 Glucose Challenge Test (GCT)
- Purpose and rationale:
- The 50-g oral glucose challenge test is a screening test designed to identify women who need definitive diagnostic testing (OGTT). It screens primarily for postprandial glucose dysregulation and is convenient because it does not require fasting.
- Test procedure (standardized steps):
- Patient does not need to fast for the 50-g GCT; however, standard diet in preceding days is advisable (see Nursing Insights).
- Administer 50 g of oral glucose solution; start timer at ingestion completion.
- Draw a venous blood sample exactly at 1 hour post ingestion to measure plasma glucose. Do not use fingerstick unless validated by laboratory protocol.
- Interpret using institution threshold (common thresholds: ≥130 mg/dL, ≥135 mg/dL, or ≥140 mg/dL). Lower thresholds increase sensitivity but increase false positives; many institutions use 140 mg/dL as a balance.
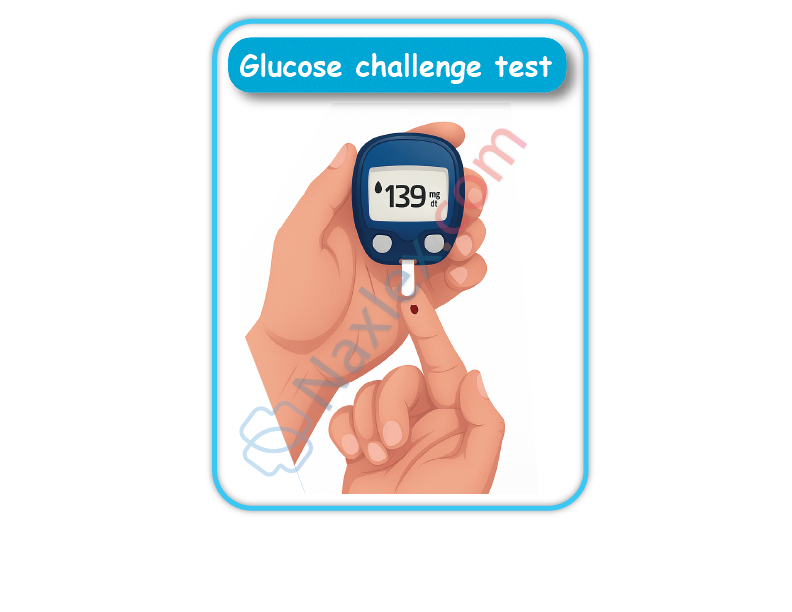
- Advantages and limitations:
- Advantage: patient convenience (non-fasting), low resource burden.
- Limitation: not diagnostic—requires follow-up OGTT if positive; sensitivity and specificity vary by threshold and population.
Nursing Insights
- Be precise about timing: the 1-hour draw must be accurate; documenting the exact ingestion and blood draw times prevents misinterpretation.
- Warn patients about possible nausea when drinking concentrated glucose solution and provide strategies (sip slowly if tolerated).
- If the patient is vomiting shortly after ingestion, the test must be repeated; document occurrence and reason for repeat.
- Instruct patients to avoid smoking or vigorous activity during the hour between ingestion and blood draw because both can alter glucose measurements.
1.3 Oral Glucose Tolerance Test (OGTT)
- Indications:
- Per the two-step approach, indicated when the 50-g GCT is above the institutional cutpoint.
- Per the one-step approach, the 75-g OGTT is the primary diagnostic test at 24–28 weeks (or earlier if high risk / abnormal fasting glucose).
- Also indicated postpartum (75-g OGTT) to detect persistent diabetes.
- Preparation for OGTT:
- Fasting: patient should fast for 8–14 hours prior to the test; only water is permitted.
- Diet: recent evidence and many guidelines recommend consuming a usual diet containing adequate carbohydrates for at least 3 days prior to the test to avoid false positives due to carbohydrate restriction.
- Medications: review medications that may affect glucose (e.g., corticosteroids, beta-blockers) and coordinate testing around such exposures when possible.
- Activity and smoking: advise avoidance of vigorous activity and smoking before and during the test.
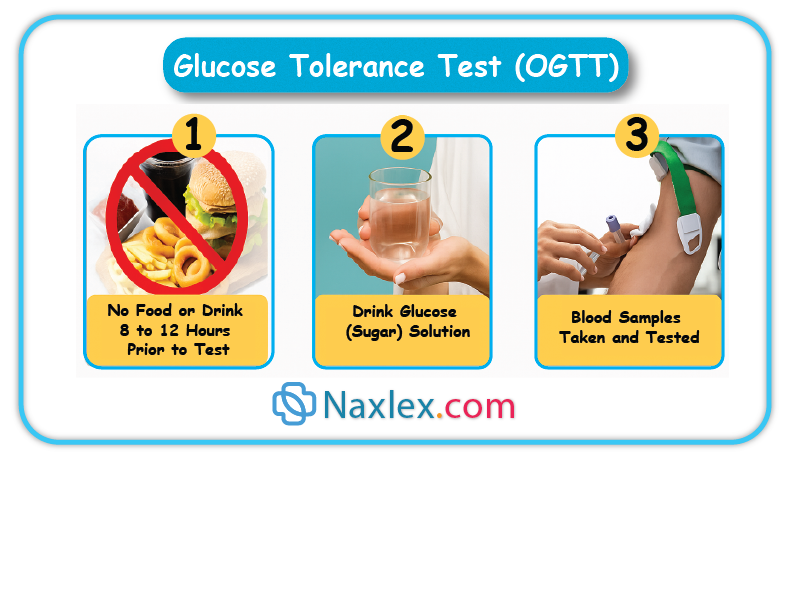
- Test types and procedures:
- 75-g 2-hour OGTT (One-step): fasting venous glucose drawn, administer 75 g glucose, then draw at 1 hour and 2 hours. Used for both diagnosis and postpartum evaluation (75 g postpartum).
- 100-g 3-hour OGTT (Two-step diagnostic test): fasting venous glucose drawn, administer 100 g glucose, then draw at 1 hour, 2 hours, and 3 hours. Diagnostic thresholds differ by Carpenter-Coustan vs older NDDG criteria.
- Common diagnostic threshold sets (widely used):
- IADPSG / WHO (One-step 75-g OGTT) — diagnostic if any one of the following is met or exceeded:
- Fasting ≥ 92 mg/dL
- 1-hour ≥ 180 mg/dL
- 2-hour ≥ 153 mg/dL
- Carpenter-Coustan (100-g OGTT, Two-step) — GDM diagnosed if ≥2 values meet or exceed:
- Fasting ≥ 95 mg/dL
- 1-hour ≥ 180 mg/dL
- 2-hour ≥ 155 mg/dL
- 3-hour ≥ 140 mg/dL
- NDDG (older) thresholds use slightly higher cutpoints (e.g., fasting 105 mg/dL); many centers still reference Carpenter-Coustan.
- IADPSG / WHO (One-step 75-g OGTT) — diagnostic if any one of the following is met or exceeded:
Table — OGTT Types and Common Diagnostic Cutpoints
|
Test |
Timing |
Common Diagnostic Cutpoints |
|
75-g OGTT (IADPSG/WHO) |
Fasting, 1h, 2h |
Fasting ≥92 mg/dL; 1h ≥180 mg/dL; 2h ≥153 mg/dL; any one abnormal = GDM |
|
100-g OGTT (Carpenter-Coustan) |
Fasting, 1h, 2h, 3h |
Fasting ≥95 mg/dL; 1h ≥180 mg/dL; 2h ≥155 mg/dL; 3h ≥140 mg/dL; ≥2 abnormal = GDM |
Nursing Insights
- Preparation is critical: ensure patient has fasted 8–14 hours and had normal carbohydrate intake in prior days. If the patient fasted but had inadequate carbohydrate intake in prior days, consider rescheduling or document potential for false results.
- Strict timing of blood draws and accurate sample handling are essential; label tubes clearly and communicate timing to lab staff.
- Be aware that different diagnostic criteria exist; when communicating results to the patient and providers, clearly state which criteria were used (e.g., “75-g OGTT, IADPSG criteria applied”).
- For the 100-g OGTT, instruct patients to plan for a clinical visit of at least 3 hours and provide a comfortable setting; consider antiemetic measures if prior nausea with concentrated glucose.
1.4 Diagnostic Criteria
- Interpretation depends on the chosen approach: the nurse must know which criteria apply locally and document them clearly in the chart. Below are operational rules for interpretation.
- 75-g OGTT (IADPSG/WHO; One-step): diagnosis of GDM is made if any one value meets or exceeds the threshold (fasting ≥92 mg/dL; 1-hour ≥180 mg/dL; 2-hour ≥153 mg/dL). This approach increases sensitivity and identifies women with milder abnormalities associated with adverse outcomes.
- 100-g OGTT (Carpenter-Coustan; Two-step diagnostic): diagnosis is made if two or more values meet or exceed thresholds (fasting ≥95 mg/dL; 1-hour ≥180 mg/dL; 2-hour ≥155 mg/dL; 3-hour ≥140 mg/dL). Use of Carpenter-Coustan criteria is common in centers using the two-step approach.
- Overt Diabetes vs GDM:
- If early pregnancy testing (first prenatal visit) shows A1c ≥6.5% or fasting glucose ≥126 mg/dL or random glucose ≥200 mg/dL with symptoms, the patient is considered to have overt diabetes (preexisting) and should be managed accordingly; do not label as GDM.
- Borderline or discordant results:
- If one test is borderline or if clinical suspicion is high despite negative screening, consider repeat testing, early postpartum testing, or referral to endocrinology as appropriate. Clinical judgment is required for cases with discordant results and multiple comorbidities.
Nursing Insights
- When communicating results, always specify the test type, numeric values, and which diagnostic criteria were applied; e.g., “75-g OGTT: fasting 94 mg/dL, 1h 178 mg/dL, 2h 160 mg/dL — IADPSG criteria: 2-hour abnormal → GDM.” Clear documentation prevents misclassification.
- Educate patients on the distinction between overt diabetes and GDM to reduce anxiety and ensure appropriate follow-up; explain that overt diabetes requires more intensive management.
- For borderline abnormal results, create a follow-up plan: increased SMBG, repeat OGTT, or referral within days to weeks depending on clinical risk.
1.5 Postpartum Screening For Persistent Glucose Intolerance
- Rationale: GDM frequently resolves after delivery, but women with GDM are at substantially higher lifetime risk of Type 2 diabetes; postpartum testing identifies persistent diabetes or prediabetes and permits early preventive measures.
- Recommended timing and tests:
- 6–12 weeks postpartum: perform a 75-g 2-hour OGTT (preferred) to detect persistent diabetes or impaired glucose tolerance. This timing allows for return toward baseline insulin sensitivity and a reliable assessment of glucose metabolism.
- Alternative or adjunct tests: fasting plasma glucose and hemoglobin A1c can be used but are less sensitive for detecting impaired glucose tolerance in the early postpartum period; if using A1c, interpret cautiously in women with recent blood loss or transfusion.
- Subsequent screening: if postpartum OGTT is normal, repeat screening for diabetes at least every 1–3 years (frequency individualized by other risk factors). Annual screening may be appropriate for women with multiple risk factors (obesity, strong family history).
- Long-term follow-up and prevention:
- Counsel and support lifestyle interventions: weight reduction if overweight, regular physical activity, and dietary modifications to prevent progression to Type 2 diabetes. Breastfeeding is encouraged and associated with improved maternal glucose metabolism.
- Consider referral to primary care or endocrinology for ongoing surveillance and risk management; discuss use of metformin for diabetes prevention in selected high-risk women per clinical guidelines.
Nursing Insights
- Arrange postpartum OGTT prior to hospital discharge planning: schedule testing for 6–12 weeks and provide reminder systems (calls/texts) because many women do not return for postpartum care.
- Educate patients about the high risk of progression to Type 2 diabetes and the importance of postpartum screening and lifestyle changes; provide written, culturally relevant materials and local resources (nutritionists, community exercise programs).
- Coordinate care transitions: ensure a documented handoff to primary care with results and a plan for long-term screening; consider electronic reminders in the chart.
Maternal And Fetal Complications Of Diabetes In Pregnancy
Diabetes in pregnancy predisposes both the mother and fetus to a spectrum of pathophysiologic derangements that arise from maternal hyperglycemia, fetal hyperinsulinemia, altered placental function, and associated comorbidities. The following sections examine maternal complications and fetal/neonatal complications in depth, with mechanistic explanations, clinical manifestations, diagnostic considerations, and nursing implications.
1.1 Maternal Complications
1.1.1 Diabetic Ketoacidosis (DKA)
- Pathophysiology and precipitating factors
- Diabetic ketoacidosis is an acute, life-threatening decompensation characterized by absolute or relative insulin deficiency, marked hyperglycemia, increased lipolysis, ketogenesis with accumulation of ketone bodies (β-hydroxybutyrate, acetoacetate), metabolic acidosis (anion gap), and osmotic diuresis.
- Pregnancy increases susceptibility to DKA at lower glucose thresholds due to placental hormones (hPL, cortisol) that enhance insulin resistance and due to increased maternal respiratory alkalosis (which shifts the bicarbonate buffer). Common precipitants include infection, vomiting, missed insulin doses, corticosteroid administration, and intercurrent illness.
- Clinical features in pregnancy
- Maternal: polyuria, polydipsia, dehydration, nausea, vomiting, abdominal pain, Kussmaul respirations, altered mental status, hypotension, tachycardia.
- Fetal: uterine hypoperfusion and fetal distress can occur rapidly because maternal acidosis and hypovolemia reduce uteroplacental perfusion; intrauterine fetal demise is a known complication of severe DKA.
- Diagnosis
- Laboratory: plasma glucose often >200 mg/dL but may be lower in pregnancy; serum β-hydroxybutyrate elevated; arterial or venous blood gas showing high anion gap metabolic acidosis; serum electrolytes with variable potassium (initially normal/high then total body potassium depletion), elevated BUN/creatinine if volume depleted. Urinalysis positive for ketones (but serum measurement preferred).
- Note: in pregnancy, DKA can occur with modest hyperglycemia (e.g., 200–300 mg/dL) compared with nonpregnant thresholds; maintain high suspicion.
- Management principles
- Immediate resuscitation: secure airway, oxygen as needed, aggressive IV fluid resuscitation with isotonic crystalloid (e.g., 0.9% NaCl) to restore intravascular volume and perfusion.
- Insulin therapy: continuous IV regular insulin infusion after initial fluid resuscitation (bolus and infusion per protocol) to suppress ketogenesis and reduce glucose gradually; avoid rapid osmolar shifts.
- Electrolyte repletion: anticipate hypokalemia with insulin therapy; monitor serum potassium and replace promptly to maintain safe ranges (typically K+ target 4–5 mEq/L).
- Correct acidosis and underlying precipitants (e.g., treat infection, antiemetics for vomiting).
- Fetal monitoring: continuous fetal heart rate monitoring for viable fetuses after maternal stabilization; do not proceed to emergent cesarean for abnormal FHR until maternal resuscitation has been attempted unless nonreassuring FHR persists despite stabilization.
- Prognosis and prevention
- With prompt recognition and aggressive management, maternal and fetal outcomes improve; however, delays increase risk of fetal demise. Prevention includes strict adherence to insulin regimens, sick-day rules (adjust insulin, maintain hydration, contact provider early), and prompt treatment of infections.
Nursing Insights
- Recognize atypical presentation: in pregnancy, DKA may present with lower glucose values; therefore, do not rely solely on an absolute glucose threshold to exclude DKA. Act on clinical symptoms (nausea/vomiting, abdominal pain, tachypnea) with prompt bedside glucose and ketone assessment.
- Implement sick-day protocols: educate patients to check ketones when ill or hyperglycemic (>250 mg/dL), to not stop insulin, to hydrate with carbohydrate-containing fluids as advised, and to seek immediate care for persistent vomiting or positive ketones.
- In acute management, the nurse must vigilantly monitor fluid input/output, hourly glucose, insulin infusion parameters, cardiac telemetry, and frequent electrolyte checks, with particular attention to potassium replacement timing relative to insulin infusion.
- Coordinate maternal-fetal monitoring: after maternal stabilization begins, ensure continuous FHR monitoring for viable fetus and notify obstetric team of any persistent nonreassuring tracings; document maternal interventions and fetal responses precisely.
1.1.2 Preeclampsia
- Pathophysiologic link to diabetes
- Diabetes (especially preexisting T2DM with chronic vascular disease) increases risk of gestational hypertension and preeclampsia due to endothelial dysfunction, chronic inflammation, preexisting hypertension, and microvascular disease. Hyperglycemia promotes oxidative stress and impaired nitric oxide–mediated vasodilation, augmenting preeclampsia risk.
- Clinical implications
- Pregnant women with diabetes have higher incidence of severe preeclampsia, earlier onset disease, and increased maternal complications (eclampsia, HELLP syndrome). Concomitant nephropathy increases risk for superimposed preeclampsia and worsened renal outcomes.
- Diagnosis and surveillance
- Standard diagnostic criteria for preeclampsia apply (new-onset hypertension ≥140/90 mm Hg with proteinuria or end-organ dysfunction after 20 weeks). In women with preexisting proteinuria or chronic hypertension, diagnosis of superimposed preeclampsia requires new or worsening hypertension, sudden increase in proteinuria, or new end-organ dysfunction.
- Surveillance: tighter blood pressure monitoring, renal function tests, urine protein quantification, and ophthalmologic assessment if retinopathy suspected.
Nursing Insights
- For women with diabetes, document baseline blood pressure and renal function early; institute frequent antenatal BP checks and educate patients to monitor for preeclampsia symptoms (headache, visual changes, epigastric pain, rapid edema) and to report them immediately.
- Recognize that preeclampsia in a woman with diabetic nephropathy may be difficult to differentiate; serial trends in BP, urine protein, and laboratory markers (platelets, LFTs) help clarify superimposed disease.
- Prepare for early delivery if severe preeclampsia develops; ensure neonatal team notification due to increased likelihood of prematurity and neonatal complications.
1.1.3 Polyhydramnios
- Mechanism
- Polyhydramnios (excess amniotic fluid) occurs in diabetic pregnancies predominantly due to fetal hyperglycemia → fetal osmotic diuresis → increased fetal urine output → excess amniotic fluid volume. Additional contribution may come from fetal gastrointestinal anomalies or impaired swallowing in other contexts, but in diabetes osmotic diuresis is common.
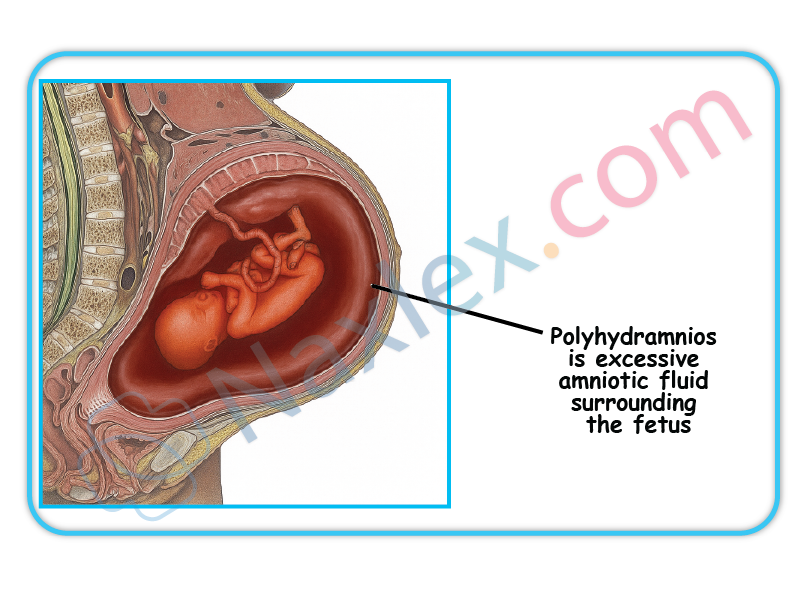
- Clinical consequences
- Increased risk of preterm labor, malpresentation, uterine overdistension, placental abruption, and postpartum hemorrhage due to uterine atony. Polyhydramnios can complicate intrapartum management and increase cesarean risk.
- Diagnosis and monitoring
- Ultrasound measurement using amniotic fluid index (AFI) or single deepest pocket (SDP) methods; AFI >25 cm or SDP >8 cm commonly used thresholds for polyhydramnios (guidelines vary). Serial ultrasounds to monitor progression.
Nursing Insights
- When polyhydramnios is identified, counsel patient about signs of preterm labor and decreased fetal movement; increase antenatal surveillance and plan for potential preterm delivery.
- Expect more intensive intrapartum management: anticipate need for tocolysis if preterm labor occurs (balance against maternal glycemic control and other contraindications) and prepare for possible postpartum hemorrhage—ensure uterotonics are available and blood loss monitoring is meticulous.
1.1.4 Infections
- Increased susceptibility and mechanisms
- Hyperglycemia impairs leukocyte function (chemotaxis, phagocytosis), impairs complement activity, and augments glucose in body fluids, providing pro-growth conditions for pathogens. Diabetic pregnancy predisposes to urinary tract infections (including asymptomatic bacteriuria progressing to pyelonephritis), vulvovaginal candidiasis, and surgical site infections postpartum or after cesarean.
- Clinical impact
- Infections can precipitate metabolic decompensation (DKA), cause preterm labor, and increase maternal morbidity. Pyelonephritis in pregnancy is associated with sepsis and preterm birth.
Nursing Insights
- Screen for and promptly treat asymptomatic bacteriuria in pregnant women with diabetes as per guidelines because of higher progression risk to pyelonephritis.
- Provide patient education on genital hygiene, recognizing signs of infection (fever, dysuria, foul-smelling discharge), and early reporting. Emphasize blood glucose optimization to reduce infection risk.
- In perioperative settings (e.g., cesarean delivery), implement strict sterile technique and glycemic control to reduce surgical site infections; coordinate appropriate antibiotic prophylaxis.
1.2 Fetal Complications
Fetal and neonatal complications arise predominantly from maternal hyperglycemia causing fetal hyperinsulinemia, abnormal growth patterns, altered organ maturation, and metabolic instability postdelivery.
1.2.1 Congenital Anomalies
- Epidemiology and risk factors
- Preexisting maternal diabetes (especially poor glycemic control during organogenesis, i.e., first trimester) increases the risk of structural congenital anomalies—most commonly congenital cardiac defects (septal defects, conotruncal anomalies), neural tube defects (spina bifida), and caudal regression spectrum. GDM diagnosed after the first trimester is less commonly associated with congenital anomalies unless preexisting diabetes was unrecognized.
- Pathophysiology
- Hyperglycemia during embryogenesis induces oxidative stress, alters gene expression, and interferes with morphogenetic signaling pathways, increasing malformation risk. Elevated A1c in early pregnancy strongly correlates with increased malformation rates.
- Screening and prevention
- Preconception glycemic optimization (A1c target individualized, commonly <7% if safely achievable) reduces anomaly risk. Early anatomic ultrasound (dating and targeted anatomy scan) and first-trimester nuchal translucency and cell-free DNA screening are used per obstetric guidelines.
Nursing Insights
- Counsel women with preexisting diabetes about the critical importance of preconception A1c optimization and early first-trimester glycemic control to reduce congenital anomaly risk.
- Facilitate early referral for high-resolution fetal anatomic ultrasound and maternal–fetal medicine consultation when pregestational diabetes or early hyperglycemia is present.
1.2.2 Macrosomia
- Definition and mechanism
- Fetal macrosomia is often defined as birth weight >4,000 g or >4,500 g depending on context; large-for-gestational-age (LGA) refers to birth weight >90th percentile for gestational age. Maternal hyperglycemia → increased transplacental glucose → fetal hyperglycemia → fetal pancreatic β-cell hypertrophy and hyperinsulinemia → insulin-mediated somatic growth and adipose deposition → macrosomia.

- Complications
- Increased risk of shoulder dystocia, brachial plexus injury, clavicle fracture, birth trauma, perineal lacerations, postpartum hemorrhage, and cesarean delivery. Long-term risks include childhood obesity and metabolic syndrome.
- Prediction and management
- Ultrasound estimation of fetal weight in the third trimester can suggest macrosomia but has limited accuracy. Clinical decisions regarding mode of delivery consider estimated fetal weight, maternal pelvis, prior obstetric history, and comorbidities. Tight maternal glycemic control, particularly limiting postprandial hyperglycemia, reduces macrosomia risk.
Nursing Insights
- Anticipate shoulder dystocia in labor for macrosomic infants: prepare necessary equipment (e.g., neonatal resuscitation, shoulder dystocia pack), ensure team readiness, and document maneuvers if dystocia occurs.
- Educate diabetic pregnant patients on the role of postprandial glucose control in preventing excess fetal growth; reinforce SMBG and MNT adherence.
1.2.3 Intrauterine Growth Restriction (IUGR)
- Etiology in diabetic pregnancies
- IUGR may occur particularly in women with long-standing pregestational diabetes complicated by vascular disease (placental insufficiency due to diabetic vasculopathy), maternal hypertension, or nephropathy. IUGR in diabetes is thus usually associated with maternal vascular disease, unlike macrosomia which is associated with maternal hyperglycemia without significant vascular compromise.
- Clinical significance
- IUGR increases risks of oligohydramnios, stillbirth, perinatal morbidity, and long-term neurodevelopmental sequelae. Management focuses on close fetal surveillance and timely delivery when intrauterine environment is unsafe.
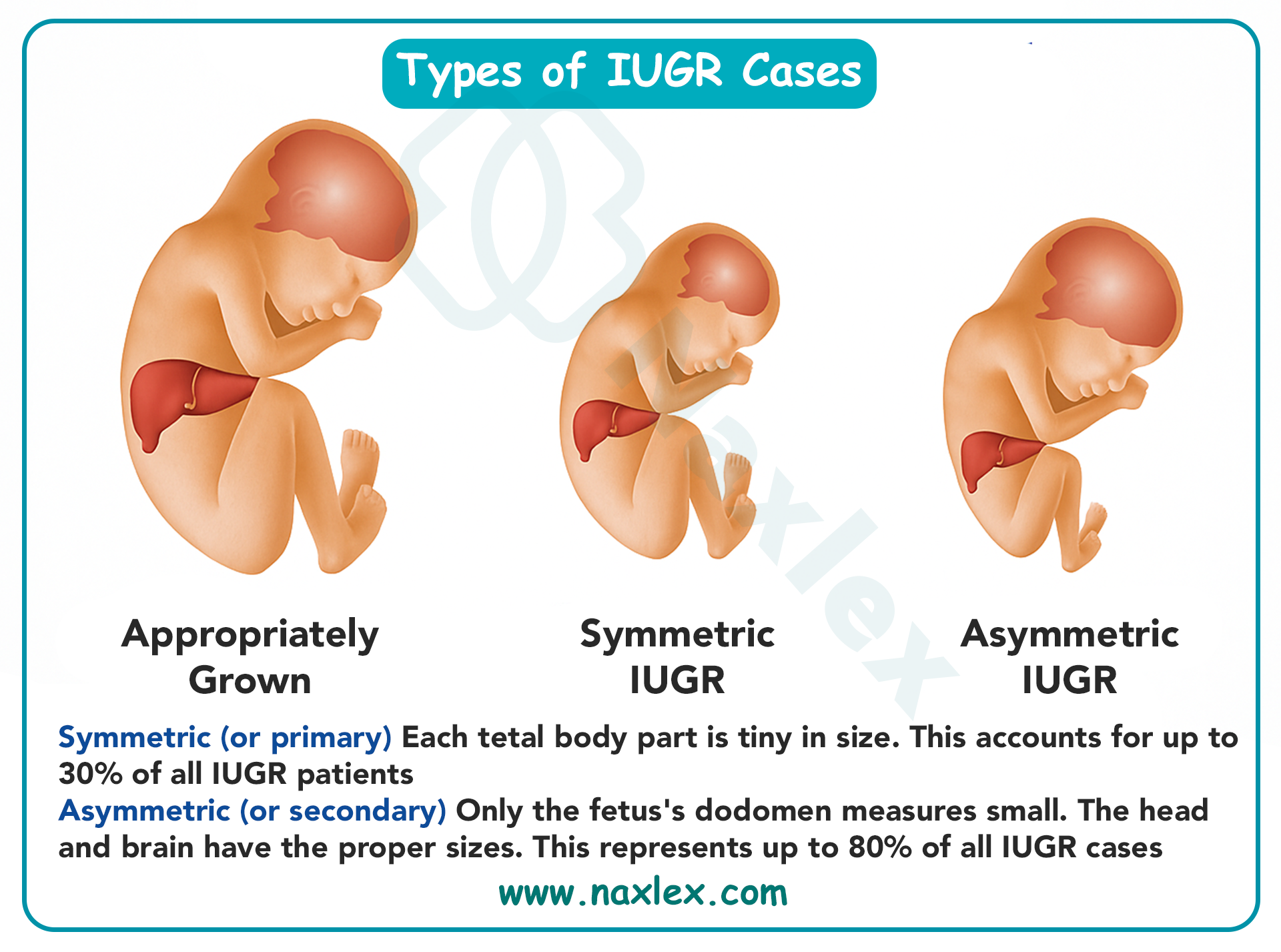
Nursing Insights
- Identify patients with microvascular disease (retinopathy, nephropathy) as higher risk for IUGR; schedule serial growth ultrasounds and Doppler studies as indicated.
- Educate such patients that optimal blood pressure and glycemic control reduce placental insufficiency progression and that antenatal surveillance is essential.
1.2.4 Respiratory Distress Syndrome (RDS)
- Mechanism
- Fetal hyperinsulinemia antagonizes cortisol-stimulated surfactant production and type II pneumocyte maturation, delaying lung maturity even when the fetus is at or near term. This biochemical interference increases the risk of RDS in infants of diabetic mothers (IDMs).
- Clinical presentation and risk factors
- Tachypnea, nasal flaring, retractions, grunting, hypoxemia soon after birth. Risk is greater with poor maternal glycemic control, prematurity, and fetal hyperinsulinemia.
- Prevention and management
- Optimize maternal glycemic control antenatally. For preterm deliveries or high RDS risk, antenatal corticosteroids may be administered according to obstetric guidelines (weigh benefits vs potential hyperglycemic effects). Neonatal management includes CPAP, supplemental oxygen, surfactant administration as indicated, and NICU support.
Nursing Insights
- Anticipate possible respiratory support for neonates of diabetic mothers even if delivery occurs at term; ensure neonatal team present at delivery and prepare equipment for immediate respiratory stabilization.
- Monitor neonate closely in the immediate postnatal period for respiratory distress signs and coordinate early blood glucose checks due to concurrent risk of hypoglycemia.
1.2.5 Neonatal Hypoglycemia
- Pathophysiology
- After delivery, the neonate’s continuous maternal glucose supply ceases abruptly while neonatal hyperinsulinemia (from in utero exposure to maternal hyperglycemia) persists, causing rapid decline in neonatal blood glucose and hypoglycemia. Risk is higher when maternal hyperglycemia is poorly controlled in late pregnancy or when infants are macrosomic.
- Clinical features
- Jitteriness, lethargy, poor feeding, apnea, temperature instability, seizures in severe cases. Neonatal hypoglycemia can lead to neuronal injury if severe and prolonged.
- Screening and management
- Early feeding (breast or formula) within 30–60 minutes of birth is recommended for infants at risk; frequent glucose monitoring per protocol (e.g., within 30–60 minutes of birth, then at 2–4 hours and before feeds as needed). Thresholds for intervention vary by institution (common action thresholds: <40–45 mg/dL in first 24 hours), and management includes enteral feeding, oral glucose gel, and intravenous dextrose (e.g., D10W bolus followed by infusion) for refractory or severe hypoglycemia.
Nursing Insights
- Implement early and proactive glucose monitoring for infants of diabetic mothers: ensure first glucose check within 30–60 minutes after birth and before next feed. Document timing of feeds and glucose values precisely.
- Educate parents prenatally about risk of neonatal hypoglycemia, the importance of early feeding, and possible need for NICU observation if hypoglycemia occurs.
- For symptomatic neonates or persistent low glucose, prepare to initiate IV glucose therapy and coordinate with neonatal team for further stabilization and neuroprotective measures.
Table — Maternal vs Fetal/Neonatal Complications (Concise Comparison)
|
Maternal Complications |
Mechanism / Clinical Notes |
Fetal/Neonatal Complications |
Mechanism / Clinical Notes |
|
Diabetic ketoacidosis (DKA) |
Insulin deficiency → ketogenesis; precipitated by infection, vomiting |
Congenital anomalies |
Early hyperglycemia → teratogenesis (cardiac, neural tube) |
|
Preeclampsia |
Endothelial dysfunction, microvascular disease |
Macrosomia/LGA |
Fetal hyperinsulinemia → adipogenesis |
|
Polyhydramnios |
Fetal osmotic diuresis from hyperglycemia |
IUGR |
Maternal vascular disease → placental insufficiency |
|
Infection (UTI, candidiasis) |
Hyperglycemia impairs immunity |
Respiratory distress syndrome (RDS) |
Fetal hyperinsulinemia delays surfactant synthesis |
|
— |
— |
Neonatal hypoglycemia |
Abrupt loss of maternal glucose + persistent fetal insulin |
Management Of Diabetes In Pregnancy
Comprehensive management of diabetes in pregnancy integrates preconception optimization, individualized medical nutrition therapy, exercise, vigilant glucose monitoring, safe pharmacotherapy when required, and multidisciplinary peripartum and postpartum planning. The goal is to maintain maternal euglycemia within pregnancy-specific targets to minimize maternal and fetal complications while preserving maternal safety.
1.1 Preconception Counseling And Glycemic Control
- Purpose and rationale:
- Preconception counseling reduces the risk of congenital anomalies, spontaneous abortion, and other adverse outcomes by achieving stable glycemic control during organogenesis (first 8–10 weeks).
- Counseling addresses medication safety, optimization of chronic comorbidities (hypertension, renal disease, retinopathy), immunizations, weight optimization, and psychosocial readiness.
- Key elements of preconception management:
- Document and review recent A1c (goal individualized; many programs target ≤7% if achievable without significant hypoglycemia; tighter targets such as <6.5% may be considered in selected patients with close monitoring).
- Review and adjust medications: discontinue teratogenic agents (e.g., statins, certain ACE inhibitors) and arrange transition to pregnancy-safe alternatives; plan insulin initiation or adjustment as needed.
- Optimize blood pressure with pregnancy-safe antihypertensives if indicated (coordinate with obstetric/primary care).
- Screen for and document baseline microvascular complications (ophthalmology for retinopathy, urine albumin/creatinine and serum creatinine for nephropathy).
- Provide individualized family-planning counseling and folic acid supplementation (higher-dose folic acid for women with pregestational diabetes or prior neural tube defect).
Nursing Insights
- Obtain and record the last A1c value in the chart and counsel the woman on target ranges and risks of elevated A1c during early pregnancy; schedule follow-up visits to titrate therapy preconception.
- Review all current medications and provide a clear written plan for which drugs to stop and which to continue; confirm understanding and document patient acknowledgement.
- Facilitate referrals (endocrinology, MFM, ophthalmology) before conception; proactively schedule these so the patient completes evaluations prior to pregnancy.
1.2 Medical Nutrition Therapy (MNT)
- Principles and goals:
- MNT aims to achieve euglycemia, appropriate gestational weight gain, and adequate fetal nutrition. It individualizes caloric needs based on pre-pregnancy BMI while focusing on macronutrient distribution that prevents large postprandial glycemic excursions.
- Caloric needs and distribution:
- Energy requirements should be individualized:
- Underweight (BMI <18.5): modest caloric increase above baseline.
- Normal weight (BMI 18.5–24.9): additional ~300 kcal/day during 2nd and 3rd trimesters is often recommended.
- Overweight/obese (BMI ≥25): restrict excessive caloric excess; individualized calorie prescription aiming for appropriate gain per IOM guidelines.
- Macronutrient distribution (example evidence-based framework):
- Carbohydrates: ~40–50% of total daily calories with emphasis on complex carbohydrates and fiber.
- Protein: 20–25% of calories (sufficient to support fetal growth).
- Fat: 25–35% of calories emphasizing unsaturated fats and limiting saturated/trans fats.
- Meal pattern: 3 balanced meals + 2–3 snacks (including bedtime snack if needed) to avoid fasting hypoglycemia and reduce postprandial peaks.
- Energy requirements should be individualized:
- Glycemic carbohydrate management:
- Emphasize low glycemic index carbohydrates, high fiber, and even distribution of carbohydrate across meals rather than large boluses.
- Avoid sugar-sweetened beverages and high-simple-sugar intake. Encourage portion control and carbohydrate counting for patients on insulin.
- Micronutrients and other considerations:
- Ensure adequate folate, iron, calcium, vitamin D, and other prenatal micronutrients per standard prenatal guidelines.
- Tailor MNT to cultural food preferences and socioeconomic realities.
Table — Example Daily Meal Distribution (Illustrative)
|
Meal |
Carbohydrate Strategy |
Notes |
|
Breakfast |
Moderate carbohydrate bolus with protein/fat to slow absorption |
Avoid high-sugar cereals; choose whole grains |
|
Midmorning snack |
Small carbohydrate + protein |
Prevents early hypoglycemia |
|
Lunch |
Balanced carbohydrate + protein + vegetables |
Emphasize complex carbs |
|
Afternoon snack |
Similar to midmorning |
Useful for glycemic stabilization |
|
Dinner |
Balanced with attention to evening postprandial levels |
Monitor bedtime glucose |
|
Bedtime snack (if needed) |
Protein + small carbohydrate |
Prevents nocturnal hypoglycemia |
Nursing Insights
- Provide concrete meal examples and portion visuals rather than abstract percentages; e.g., "1 slice whole-grain bread or 1/2 cup cooked rice = ~15 g carbohydrate."
- Teach carbohydrate counting basics and how to adjust insulin (if on insulin) relative to carbohydrate intake.
- Reinforce that MNT is medical treatment: document nutrition goals in the plan of care and coordinate referral to a registered dietitian specializing in diabetes and pregnancy.
1.3 Physical Activity And Exercise Recommendations
- Rationale:
- Exercise increases insulin-mediated glucose uptake in skeletal muscle, improves insulin sensitivity, supports weight management, and reduces GDM risk/severity.
- Recommended activity:
- For uncomplicated pregnancies, advise moderate-intensity aerobic activity such as brisk walking, swimming, or stationary cycling for ≥150 minutes/week (e.g., 30 minutes on most days), unless contraindicated.
- Strength training and pelvic floor exercises may be included with appropriate supervision. Avoid high-impact contact sports or activities with high fall risk.
- Precautions:
- Contraindications include maternal cardiac/pulmonary disease, premature labor risk, uncontrolled preeclampsia, or other obstetric restrictions. Screen for contraindications before recommending exercise.
- Monitor for symptoms of hypoglycemia during/after exercise in women on insulin; advise a small carbohydrate snack if glucose trending low.
Nursing Insights
- Provide prescribed, written exercise plans individualized to fitness level and cultural context; document exercise counseling and patient acceptance.
- Teach SMBG actions around exercise: check glucose before, during (if prolonged), and after exercise; adjust carbohydrate intake or insulin per individualized plan.
1.4 Pharmacological Management
Pharmacologic therapy is indicated when MNT and exercise do not achieve glycemic targets. Insulin remains the preferred pharmacotherapy for pregnancy due to its efficacy and lack of placental transfer; certain oral agents are used selectively with informed counseling.
1.4.1 Insulin Therapy
- Rationale and advantages:
- Insulin does not cross the placenta, allows precise titration (basal and bolus), and is effective at achieving euglycemia; therefore it is the gold standard in pregnancy when pharmacologic therapy is required.
- Types of insulin and typical roles:
- Rapid-acting analogs: insulin lispro and aspart — used for premeal/bolus control due to rapid onset and shorter duration.
- Short-acting regular insulin: alternative for prandial coverage, but slower onset and longer peak than rapid analogs.
- Intermediate-acting: NPH — traditionally used as basal coverage in split-mix regimens.
- Long-acting analogs: detemir and glargine — used as basal insulin in some patients; evidence supports safety and efficacy but practice varies by institution.
- Insulin pumps (CSII): appropriate for motivated, experienced patients with Type 1 diabetes; requires specialized support.
- Regimen strategies:
- Basal-bolus (preferred): long-acting basal insulin + rapid-acting bolus insulin with meals to mimic physiologic insulin.
- Split-mixed: premixed insulin preparations 2–3 times daily may be used in some settings but allow less flexibility.
- Titration principles: adjust based on SMBG results, focusing on fasting and postprandial targets; increase basal insulin for elevated fasting glucose, increase bolus insulin for postprandial hyperglycemia.
- Glycemic targets commonly used in pregnancy: (document which institutional targets are used)
- Fasting (preprandial) ≤95 mg/dL.
- 1-hour postprandial ≤140 mg/dL or 2-hour postprandial ≤120 mg/dL (use one convention consistently per institution).
- Individualize targets for patients at risk of hypoglycemia.
- Practical dosing considerations:
- Insulin requirements typically decrease in early pregnancy, then rise in the 2nd and 3rd trimesters (may double/triple by late pregnancy in some patients).
- Monitor for and manage hypoglycemia, especially nocturnal episodes; provide sick-day insulin adjustment education.
- During labor, IV insulin infusion protocols are commonly used to maintain intrapartum euglycemia (see Section 12.6).
Nursing Insights
- Teach patients injection technique, site rotation, storage, and hypoglycemia recognition/treatment; assess competency with return demonstration.
- Maintain clear orders for insulin adjustment rules: e.g., if fasting glucose >95 on 2 consecutive days, increase basal insulin by X% or Y units (institution-specific). Document and follow protocols rather than ad hoc changes.
- For patients on insulin pumps, coordinate with endocrinology for pump management during labor and anesthesia; ensure backup subcutaneous or IV insulin orders are available.
1.4.2 Oral Hypoglycemics (Metformin, Glyburide)
- Metformin:
- Mechanism: decreases hepatic gluconeogenesis and improves peripheral insulin sensitivity.
- Placental transfer: metformin crosses the placenta; long-term effects remain under investigation though many studies support its relative safety and efficacy in GDM and in women with T2DM or PCOS.
- Clinical use: used as monotherapy or adjunct in selected women, particularly where insulin access is limited, or to reduce maternal weight gain; used with counseling about placental transfer and potential need for supplemental insulin if glycemic targets are not met.
- Glyburide (glibenclamide):
- Mechanism: sulfonylurea that stimulates insulin secretion.
- Placental transfer: older data suggested minimal placental transfer, but more recent studies show measurable transfer; Glyburide may increase fetal insulin and risk of neonatal hypoglycemia in some settings.
- Clinical use: used in some centers for GDM when patients decline insulin, with awareness of variable efficacy and neonatal considerations.
- Clinical considerations and caveats:
- Insulin remains the preferred first-line pharmacotherapy for most pregnant patients requiring medication due to its established safety profile.
- If oral agents are used, counsel the patient thoroughly about risks/benefits, monitor closely, and be prepared to escalate to insulin if glycemic targets are not achieved.
Nursing Insights
- When oral agents are used, ensure informed consent documentation and provide clear instructions regarding SMBG frequency and indications for switching to insulin.
- Monitor neonates for hypoglycemia more vigilantly when maternal oral hypoglycemics were used in late pregnancy; notify neonatal team of maternal medications.
1.5 Monitoring Blood Glucose Levels
- Self-monitoring of blood glucose (SMBG):
- Frequency: typical SMBG schedules include fasting and 1- or 2-hour postprandial checks after each meal (total 4 checks/day) for many women with GDM; women with preexisting diabetes or on insulin may perform more frequent checks (preprandial, 1-2 hour postprandial, bedtime, and occasionally overnight).
- Targets: fasting ≤95 mg/dL, 1-hour postprandial ≤140 mg/dL or 2-hour postprandial ≤120 mg/dL (adopt institutional standard).
- Documentation: patients should record values, meal content, insulin doses, and symptoms for provider review.
- Glycated hemoglobin (A1c):
- Use for preconception and early pregnancy assessment; A1c is less useful for tight day-to-day titration in pregnancy but provides long-term glycemic indicator. Interpret A1c with knowledge of physiologic changes in pregnancy and potential confounders (anemia, hemoglobinopathy).
- Continuous glucose monitoring (CGM):
- CGM may be used increasingly in pregnant women with Type 1 diabetes and selected high-risk patients to improve time-in-range and reduce hypoglycemia; CGM can guide insulin titration and detect nocturnal hypoglycemia. Institutional policies on CGM use vary.
- Point-of-care meter considerations:
- Ensure meter accuracy and appropriate calibration; provide education on proper technique (handwashing, correct sample volume, strip storage). Confirm that meters used at bedside and by patients are validated and that staff/documentation matches device readings.
Nursing Insights
- Teach patients how to respond to out-of-range SMBG values with explicit action plans (when to adjust insulin per sliding rules, when to call provider, when to seek emergency care).
- Emphasize trend interpretation: advise patients and clinicians to make insulin adjustments based on consistent patterns rather than single isolated readings.
- For hospitalized patients, implement clear glucose monitoring schedules and ensure hourly or more frequent checks if on IV insulin infusions, per protocol.
1.6 Nursing Management During Labor And Delivery
- Goals during labor:
- Maintain maternal blood glucose in a euglycemic range to minimize neonatal hypoglycemia risk while avoiding maternal hypoglycemia. Prevent large maternal-to-fetal glucose fluctuations. Facilitate safe anesthesia and surgical planning if cesarean delivery is required.
- Common intrapartum protocol elements:
- Frequent glucose monitoring (e.g., hourly) during active labor.
- For women on insulin: transition to an IV insulin infusion (regular insulin) with concomitant dextrose-containing fluids as needed to maintain target glucose, or continue subcutaneous regimens for short labors per institutional protocol.
- Typical intrapartum glucose target frequently used: 70–110 mg/dL (institution-dependent). Avoid glucose >110–140 mg/dL to reduce fetal hyperglycemia and subsequent neonatal hypoglycemia.
- For women not on insulin with well-controlled GDM, some centers monitor and treat only if glucose exceeds threshold; protocols vary.
- Perioperative considerations for cesarean delivery:
- Coordinate timing of last subcutaneous insulin dose relative to anesthesia/surgery; often switch to IV insulin infusion perioperatively to maintain tighter control and permit rapid adjustments.
- Monitor fluid balance and electrolytes; be aware of corticosteroid use (for fetal lung maturity) that will acutely raise maternal glucose.
- Neonatal preparation and communication:
- Notify neonatal/pediatric team before delivery of infants of diabetic mothers; ensure plan for early feeding, glucose monitoring, and possible NICU support if needed.
Nursing Insights
- Ensure clear orders for intrapartum insulin protocols and dextrose administration; document hourly glucose values and insulin rates meticulously.
- Prepare for potential neonatal hypoglycemia: have glucose gel, formula, or IV dextrose available and ensure neonatal team presence for high-risk deliveries.
- Educate laboring patients about the rationale for IV lines, frequent fingersticks, and possible temporary changes to their usual insulin regimen.
1.7 Postpartum Management And Follow-Up
- Immediate postpartum physiologic changes:
- After placental delivery, insulin resistance declines rapidly; insulin requirements drop precipitously in insulin-treated women. Risk for hypoglycemia increases if pre-pregnancy insulin dosing is continued unchanged.
- Immediate postpartum tasks:
- For mothers on insulin, reduce insulin dosage significantly (commonly ~20–50% reduction depending on pre-delivery requirements and breastfeeding intentions) and monitor glucose closely for the first 24–72 hours. Use inpatient glucose checks to guide dosing.
- For women who had GDM but were not previously on medications, frequent glucose monitoring in immediate postpartum is recommended to confirm euglycemia prior to discharge. Many will not require pharmacotherapy but need postpartum screening.
- Breastfeeding considerations:
- Breastfeeding improves maternal glucose metabolism and reduces postpartum weight; encourage and support lactation. Adjust insulin dosing as breastfeeding can increase insulin sensitivity and lower glucose; monitor to avoid hypoglycemia.
- Postpartum diabetes screening:
- Perform a 75-g 2-hour OGTT at 6–12 weeks postpartum to detect persistent diabetes or impaired glucose tolerance (see Section 8.5). If OGTT abnormal, arrange appropriate long-term care and discuss prevention strategies.
- If postpartum screening is normal, counsel on lifestyle modification and schedule periodic glucose screening (at least every 1–3 years, individualized).
- Long-term follow-up and prevention:
- Provide education on lifestyle interventions, weight reduction, and, when appropriate, consider referral to diabetes prevention programs. Discuss contraception and planning for subsequent pregnancies with glycemic optimization.
Nursing Insights
- Before discharge, provide explicit written and verbal instructions for glucose monitoring at home, signs of hypoglycemia, insulin adjustments (if applicable), and timing of postpartum OGTT. Schedule the 6–12 week OGTT appointment and remind the patient to attend.
- Coordinate handoff to primary care: send discharge summary documenting antepartum glycemic control, medications used in pregnancy, and recommendations for postpartum screening and follow-up.
- Emphasize breastfeeding benefits and provide practical lactation resources; educate on how breastfeeding may change medication needs and blood glucose patterns.
Summary Table — Management Components and Nursing Actions
|
Management Component |
Core Actions |
Nursing Responsibilities |
|
Preconception care |
A1c optimization, med review, referrals |
Document A1c, med changes, arrange referrals |
|
MNT |
Individualized calories, carb distribution |
Provide meal plans, RD referral, education |
|
Exercise |
Moderate activity 150 min/week |
Prescribe safe exercise, counsel on SMBG with exercise |
|
Insulin therapy |
Basal-bolus preferred, titration |
Teach injection technique, monitor SMBG, adjust doses per protocol |
|
Oral agents |
Metformin/glyburide selective use |
Counsel on risks/benefits, monitor closely |
|
Monitoring |
SMBG, A1c, CGM as indicated |
Teach SMBG, ensure documentation, trend-based adjustments |
|
Labor |
IV insulin protocols, hourly glucose |
Implement protocol, document, coordinate neonatal team |
|
Postpartum |
Reduce insulin needs, OGTT 6–12 wks |
Adjust dosing, schedule OGTT, provide discharge education |
Fetal Surveillance And Monitoring In Gdm
Effective fetal surveillance in pregnancies complicated by gestational diabetes mellitus (GDM) aims to identify fetal compromise early, guide timing of delivery, and reduce perinatal morbidity and mortality. Surveillance strategies integrate physiologic fetal testing (cardiotocography), biophysical assessment, ultrasound-based amniotic fluid assessment, and growth monitoring with Doppler evaluation when indicated. Frequency and modality are individualized based on glycemic control, pharmacologic therapy (insulin use), presence of comorbidities (preeclampsia, fetal growth abnormalities), and gestational age.
1.1 Nonstress Test (NST)
- Definition and purpose
- The nonstress test (NST) is a noninvasive antepartum fetal surveillance tool that records fetal heart rate (FHR) patterns in response to spontaneous fetal activity. The NST assesses fetal autonomic function and oxygenation indirectly by observing accelerations in FHR and baseline variability.
- Physiologic basis
- Fetal heart rate accelerations are mediated by intact fetal autonomic nervous system response to movement and reflect adequate fetal oxygenation and metabolic reserve. Loss of accelerations or reduced variability may indicate fetal hypoxia, acidemia, or neurologic depression.
- Indications in GDM
- NST is used for antepartum surveillance in women with poorly controlled GDM, women requiring insulin, and those with additional risk factors (hypertension, decreased fetal movement, oligohydramnios, preterm labor). Many institutions initiate NSTs at 32–34 weeks for insulin-treated GDM; timing varies by local protocol and risk assessment.
- Test procedure
- Apply a tocodynamometer to monitor uterine activity and a Doppler transducer to record fetal heart rate. The patient is usually seated or semi-recumbent. Continuous monitoring for 20–40 minutes is standard; if fetal sleep suspected, extend monitoring or use vibroacoustic stimulation (VAS).
- Document fetal movements, maternal position, medications given, and duration of the test.
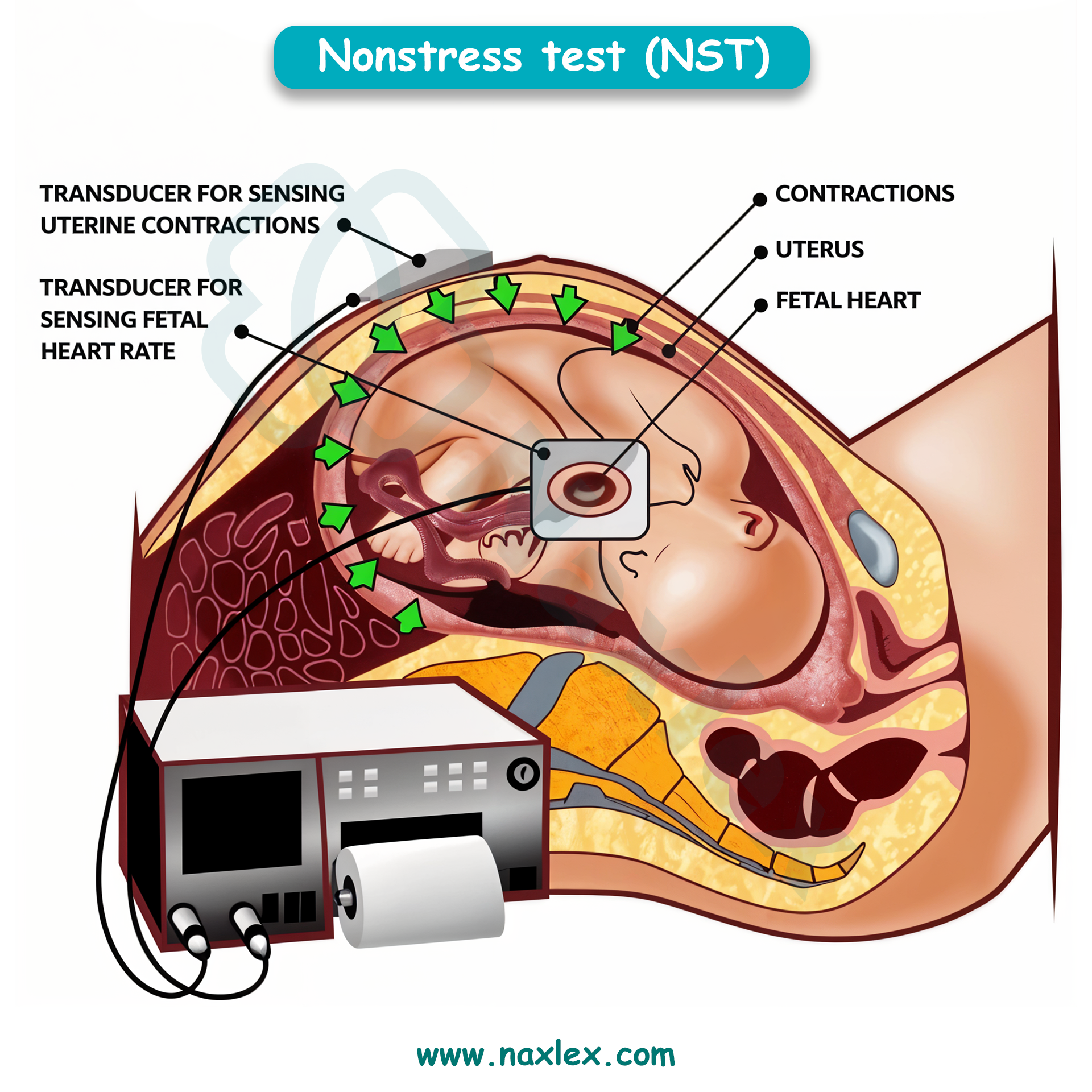
- Interpretation (standard criteria for a reactive NST)
- Reactive NST: ≥2 accelerations over baseline within 20 minutes.
- For fetuses ≥32 weeks: acceleration defined as ≥15 bpm above baseline lasting ≥15 seconds.
- For fetuses <32 weeks: acceleration defined as ≥10 bpm for ≥10 seconds.
- Nonreactive NST: fewer than required accelerations in a 40-minute period; requires further evaluation via vibroacoustic stimulation and/or biophysical profile (BPP).
- Reactive NST: ≥2 accelerations over baseline within 20 minutes.
- Vibroacoustic Stimulation (VAS)
- Used to arouse a sleeping fetus and provoke accelerations. Apply VAS for brief durations per protocol; if reactive, conclude test. If nonreactive despite VAS, proceed to BPP or extended monitoring.
- Limitations
- False positives (nonreactive despite a well fetus) due to fetal sleep cycles, maternal medications (e.g., opioids), or technician error. NST assesses only short-term fetal status and must be correlated with other testing and clinical context.
Nursing Insights
- Prepare the patient: advise her to eat a light snack prior to NST if allowed to reduce false nonreactive results due to low fetal activity; document timing of last meal.
- Accurately document test start and end times and maternal position; fetal movement events recorded by the patient should be correlated with tracings.
- If NST is nonreactive, promptly perform VAS per protocol and escalate to BPP or specialist consultation if still nonreactive—do not dismiss a nonreactive NST in a high-risk GDM pregnancy.
1.2 Biophysical Profile (BPP)
- Definition and components
- The biophysical profile integrates ultrasound assessment of fetal well-being with cardiotocography (NST) to produce a composite score reflecting fetal oxygenation and neurologic function. The standard BPP comprises 5 components, each scored 0 or 2:
- NST (reactive = 2)
- Fetal breathing movements (≥1 episode of rhythmic breathing ≥30 seconds within 30 minutes = 2)
- Gross fetal body movements (≥3 discrete body/limb movements in 30 minutes = 2)
- Fetal tone (≥1 episode of extension with return = 2)
- Amniotic fluid volume (single deepest pocket ≥2 cm or AFI >5 cm = 2)
- The biophysical profile integrates ultrasound assessment of fetal well-being with cardiotocography (NST) to produce a composite score reflecting fetal oxygenation and neurologic function. The standard BPP comprises 5 components, each scored 0 or 2:
- Scoring and interpretation
- Total score ranges 0–10.
- 8–10: reassuring (if AFI normal).
- 6: equivocal—may prompt extended monitoring, repeat testing within 24 hours, or delivery depending on gestational age and clinical context.
- ≤4: nonreassuring—often indicates potential fetal compromise; consider delivery if gestational age appropriate or intensive monitoring and further assessment.
- Total score ranges 0–10.
- Indications in GDM
- BPP is commonly used when NST is nonreactive, when fetal growth restriction or oligohydramnios is identified, or when there are additional risk factors (e.g., poor glycemic control, preeclampsia). In insulin-requiring GDM, BPP may be part of twice-weekly surveillance starting in the third trimester (institution-dependent).
- Practical procedure considerations
- Perform BPP in a calm environment; ensure maternal bladder is empty for accurate ultrasound. The full assessment may take 30–60 minutes. Document each component explicitly.
Nursing Insights
- Understand that BPP integrates multiple indicators of fetal well-being; a low BPP score in a GDM pregnancy should prompt immediate multidisciplinary discussion (obstetrician, MFM, neonatology).
- For equivocal scores (6), coordinate repeat testing and ensure maternal glucose is optimized prior to repeat testing because maternal hyperglycemia may transiently affect fetal activity patterns.
- Teach patients what BPP evaluates and the possible clinical pathways (repeat testing, induction, or cesarean) depending on results.
1.3 Amniotic Fluid Index (AFI)
- Definition and measurement
- AFI quantifies amniotic fluid using ultrasound by dividing the uterus into four quadrants and summing the deepest vertical pocket in each quadrant. An alternate method is the single deepest pocket (SDP) measurement. AFI and SDP are used to identify oligohydramnios and polyhydramnios.
- Thresholds and clinical interpretation
- AFI ≤5 cm: oligohydramnios (some centers use ≤5 cm).
- AFI 5.1–25 cm: generally normal (ranges vary).
- AFI >25 cm: polyhydramnios.
- Single deepest pocket: SDP <2 cm suggests oligohydramnios; SDP ≥8 cm suggests polyhydramnios. Institutional cutoffs may vary; clinicians should apply local guidelines.
- Relevance to GDM
- Polyhydramnios is more common in poorly controlled GDM due to fetal osmotic diuresis from hyperglycemia. Polyhydramnios increases risks for preterm labor, malpresentation, and postpartum hemorrhage. Oligohydramnios may occur with fetal growth restriction or ruptured membranes and requires different management.
- Surveillance and management
- Serial AFI monitoring is performed when abnormal volumes are detected or when maternal glycemic control is poor. Management depends on severity: mild polyhydramnios may be observed with tighter glycemic control; severe polyhydramnios may require therapeutic amnioreduction or preterm delivery if complications occur. Oligohydramnios often triggers closer surveillance and assessment of fetal status.
Nursing Insights
- When AFI abnormality is documented, ensure documentation includes method used (AFI vs SDP), numeric value, and scanner/operator. This precision guides clinical decisions.
- Counsel patients with polyhydramnios on signs of preterm labor and increased fetal movement surveillance; increase frequency of antenatal contact as indicated.
- Coordinate multidisciplinary care for severe polyhydramnios (MFM, neonatology) and prepare for potential interventions (amnioreduction, preterm delivery).
1.4 Ultrasound Evaluation Of Fetal Growth
- Biometric parameters and estimated fetal weight (EFW)
- Standard fetal biometric measures include:
- Biparietal diameter (BPD) — transverse skull diameter.
- Head circumference (HC) — skull circumference.
- Abdominal circumference (AC) — key parameter reflecting fetal adiposity and energy stores.
- Femur length (FL) — long bone measurement.
- Standard fetal biometric measures include:
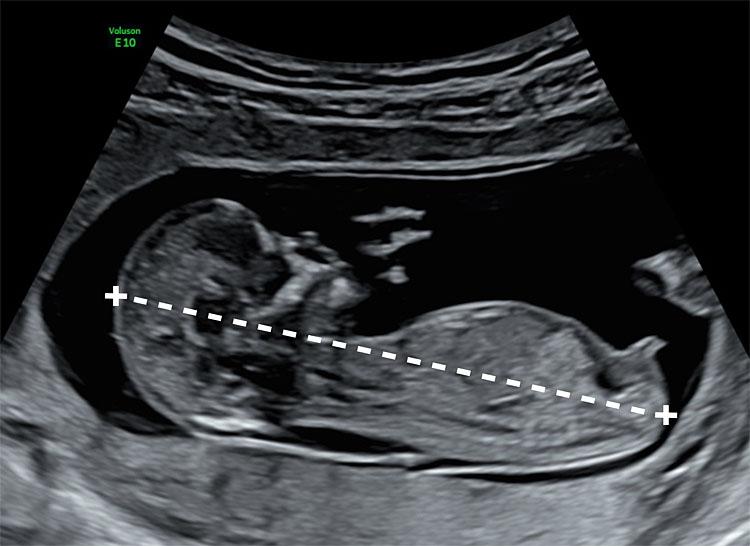
- Algorithms (Hadlock formulas) combine these measures to calculate estimated fetal weight (EFW) and percentile for gestational age.
- Timing and frequency in GDM
- For pregnancies with GDM, growth ultrasound timing is individualized: many clinicians obtain an ultrasound in the third trimester (28–32 weeks) for baseline and then serial ultrasounds every 3–4 weeks if glycemic control is suboptimal, if fetal macrosomia is suspected, or if insulin therapy is required. Frequency increases for suspected macrosomia, polyhydramnios, or maternal vascular disease (to assess for IUGR).
- Interpretation and limitations
- AC is the most sensitive parameter for fetal overgrowth in diabetic pregnancies because fetal adipose tissue accumulation is prominent.
- Ultrasound EFW has ±10–15% error margin; accuracy decreases at extremes of fetal weight (macrosomia or severe growth restriction). Maternal obesity and operator variability further reduce precision.
- Large AC percentile or rapid abdominal growth trajectory is a predictor of macrosomia; management decisions (mode/timing of delivery) integrate ultrasound estimates with clinical context.
- Doppler studies
- Indicated when placental insufficiency or IUGR is suspected. Common assessments include:
- Umbilical artery Doppler: elevated resistance/reversed end-diastolic flow indicates severe placental insufficiency.
- Middle cerebral artery (MCA): increased diastolic flow (brain-sparing) suggests fetal hypoxia.
- Ductus venosus: abnormal flow patterns indicate severe compromise.
- In GDM with vascular disease, Doppler surveillance complements growth scans to detect placental insufficiency and guide timing of delivery.
- Indicated when placental insufficiency or IUGR is suspected. Common assessments include:
- Clinical application: delivery planning
- When ultrasound suggests macrosomia (EFW >4,000–4,500 g depending on clinical context), discuss delivery options with the patient, balancing cesarean risk vs vaginal delivery with shoulder dystocia risk. Consider maternal pelvis, prior obstetric history, and estimated accuracy limitations.
- For IUGR with abnormal Dopplers, expedited delivery may be indicated despite prematurity risk, after weighing fetal maturity and neonatal support availability.
Nursing Insights
- Document EFW and percentile trend rather than single value; successive growth percentiles and abdominal circumference velocity guide clinical decisions more effectively than a one-time estimate.
- Educate mothers on the inherent measurement error of ultrasound EFW and set realistic expectations—avoid overreliance on precise weight predictions; instead, focus on risk mitigation strategies (glycemic control, intrapartum planning).
- Ensure coordination of care when abnormal growth or Dopplers are found: schedule MFM consultation, fetal surveillance (NST/BPP), and neonatal team notification for complex delivery planning.
Patient Education And Health Promotion
- Patient education and health promotion in gestational diabetes mellitus encompass multifaceted strategies to empower pregnant individuals with knowledge and skills for self-management, emphasizing blood glucose monitoring techniques to detect deviations from targets like fasting <95 mg/dL and 1-hour postprandial <140 mg/dL, recognition and prompt management of hypoglycemia and hyperglycemia to prevent neuroglycopenic symptoms or ketoacidosis, the physiological benefits of breastfeeding in enhancing maternal insulin sensitivity through prolactin-mediated glucose utilization in mammary glands, and long-term lifestyle modifications including dietary adherence and physical activity to mitigate the 50-70% lifetime risk of progression to type 2 diabetes mellitus via improved beta-cell function and reduced visceral adiposity.
- Educational approaches utilize adult learning principles, incorporating motivational interviewing to address barriers like cultural beliefs or socioeconomic constraints, with reinforcement through written materials, digital apps for glucose tracking, and group sessions to foster peer support, ultimately reducing complication rates such as macrosomia by 40% and improving postpartum glucose tolerance.
- Health promotion extends to family involvement for sustained behavioral change, with emphasis on annual screening post-GDM to detect prediabetes early, defined as impaired fasting glucose 100-125 mg/dL, allowing interventions like metformin to delay diabetes onset by 30-40%.
Nursing Insights
- Nurses deliver patient education by assessing literacy levels at initial diagnosis, using teach-back methods to confirm understanding of glucose targets, ensuring patients verbalize fasting <95 mg/dL in real clinic interactions to enhance retention and compliance.
- In group education sessions, nurses facilitate discussions on cultural meal adaptations, demonstrating low-glycemic index food choices to maintain euglycemia without restricting traditions.
1.1 Patient Teaching On Blood Glucose Monitoring
- Patient teaching on blood glucose monitoring involves comprehensive instruction on self-monitoring of blood glucose using capillary sampling with glucometers to quantify plasma glucose levels, targeting fasting values <95 mg/dL, 1-hour postprandial <140 mg/dL, and 2-hour postprandial <120 mg/dL to avert fetal hyperinsulinemia and maternal ketoacidosis in gestational diabetes mellitus.
- Techniques include hand hygiene to prevent contamination, lancet device usage for fingerstick with alternating sites to minimize neuropathy, strip insertion into calibrated meters for accurate enzymatic readings, and logging results with meal correlations to identify patterns like dawn phenomenon from overnight hepatic gluconeogenesis.
- Continuous glucose monitoring systems provide interstitial glucose trends every 5 minutes via subcutaneous sensors, alerting for hypoglycemia <70 mg/dL or hyperglycemia >180 mg/dL, with calibration twice daily against capillary values for precision within 10-15%.
- Education covers meter maintenance, error troubleshooting like insufficient sample yielding "LO" readings, and integration with apps for data sharing with providers, reducing A1c by 0.5-1% through timely adjustments.
- Step-by-step procedure: Wash hands with soap, dry thoroughly to avoid dilution errors, prick fingertip laterally to reduce pain, apply drop to strip without smearing for capillary action, read result in 5-10 seconds.
- Common errors: Expired strips causing overestimation by 20-30 mg/dL, cold hands vasoconstricting flow necessitating warm compresses.
- Frequency: 4-7 times daily including fasting, pre/post-meals, bedtime, and 3 AM if nocturnal risks.
- Advanced features: Bluetooth syncing for trend graphs showing time in range >70% between 70-140 mg/dL, predictive alarms for impending lows.
Nursing Insights
- Nurses teach blood glucose monitoring by demonstrating lancet loading and strip handling, having patients return-demonstrate to ensure proficiency, correcting errors like over-milking finger that hemolyzes samples in real teaching sessions.
- For non-English speakers, nurses use pictorial guides and interpreters, verifying understanding through simulated logs to promote accurate self-management.
Nursing Insights
- In low-resource settings, nurses prioritize affordable meters, teaching strip conservation techniques like single-use per test to sustain monitoring adherence.
1.2 Recognition And Management Of Hypoglycemia And Hyperglycemia
- Recognition of hypoglycemia in gestational diabetes mellitus involves identifying neuroadrenergic symptoms like tremors, sweating, tachycardia from counterregulatory epinephrine release when glucose <70 mg/dL, progressing to neuroglycopenic manifestations such as confusion, seizures if <50 mg/dL due to cerebral glucose deprivation.
- Management adheres to the 15-15 rule: Ingestion of 15 g fast-acting carbohydrates like glucose tablets, rechecking in 15 minutes, repeating if persistent, followed by protein-carbohydrate snack to sustain levels via delayed absorption.
- Hyperglycemia recognition includes polydipsia, polyuria from osmotic diuresis when glucose >180 mg/dL, with ketonuria indicating starvation amid insulin deficiency, risking ketoacidosis if >240 mg/dL with pH <7.3.
- Management entails correction boluses of rapid insulin 1 unit/50 mg/dL above target, hydration to dilute glucose, and activity to enhance uptake, with urine ketone checks if >200 mg/dL to guide therapy.
- Hypoglycemia triggers: Overdosing insulin, skipped meals delaying gastric emptying in pregnancy, exercise depleting glycogen without adjustment.
- Severe cases: Intramuscular glucagon 1 mg for unconsciousness, activating hepatic glycogenolysis within 10 minutes.
- Prevention: Bedtime snacks with 15-30 g carbohydrates, avoiding alcohol inhibiting gluconeogenesis.
- Hyperglycemia causes: Infection elevating cytokines like interleukin-6 impairing insulin signaling, stress hormones cortisol promoting gluconeogenesis.
- Chronic effects: Accelerated fetal adipogenesis if sustained >140 mg/dL postprandially.
- Emergency: Hospitalization for DKA with fluids, insulin drip at 0.1 units/kg/hour.
|
Condition |
Glucose Level |
Symptoms |
Immediate Management |
|
Hypoglycemia |
<70 mg/dL |
Shakiness, hunger, irritability |
15 g carbs, recheck 15 min |
|
Severe Hypoglycemia |
<50 mg/dL |
Confusion, seizure |
Glucagon IM, medical help |
|
Hyperglycemia |
>180 mg/dL |
Thirst, frequent urination |
Insulin correction, hydrate |
|
DKA |
>240 mg/dL with ketones |
Nausea, abdominal pain |
IV fluids, insulin infusion |
Nursing Insights
- Nurses teach hypoglycemia recognition by listing symptoms in handouts, role-playing scenarios where patients identify shakiness post-exercise, instructing to treat immediately with juice in daily life.
- For hyperglycemia, nurses demonstrate ketone strip usage, advising ER visit if positive and glucose >250 mg/dL to prevent fetal distress.
Nursing Insights
- In elderly GDM patients, nurses adjust teaching for visual impairments, using large-print logs and voice-activated meters to ensure accurate monitoring.
1.3 Importance Of Breastfeeding
- Breastfeeding in postpartum GDM women confers metabolic benefits by improving insulin sensitivity through prolactin-stimulated glucose uptake in lactocytes for lactose synthesis, reducing blood glucose by 5-10 mg/dL and decreasing type 2 diabetes risk by 15-20% per year of lactation via enhanced beta-cell regeneration.
- Physiological mechanisms include oxytocin release contracting myoepithelial cells for milk ejection, suppressing hypothalamic-pituitary-adrenal axis to lower cortisol, and mobilizing visceral fat stores for energy, aiding weight loss of 0.5-1 kg/month postpartum.
- Neonatal advantages encompass reduced obesity risk in offspring by 25% from bioactive factors like leptin regulating appetite, and lower infection rates from immunoglobulins, crucial in hypoglycemic neonates of diabetic mothers.
- Recommendations: Exclusive breastfeeding for 6 months, with 10-12 feeds daily to establish supply, overcoming barriers like delayed lactogenesis in GDM from insulin resistance via frequent pumping.
- Maternal benefits: Decreased ovarian and breast cancer risks by 4-10% per year lactated, improved bone mineralization postpartum.
- Challenges: Nipple pain from improper latch, mastitis risk elevated 20% in diabetics from glycosylated milk fostering bacteria.
- Support: Lactation consultants for positioning, monitoring infant weight gain >20 g/day.
- Long-term: Cumulative lactation >12 months halves diabetes progression, via epigenetic modifications enhancing insulin gene expression.
Nursing Insights
- Nurses promote breastfeeding by assessing latch in hospital, teaching hand expression for colostrum to stabilize neonatal glucose in hypoglycemic infants.
- For working mothers, nurses advise pumping schedules every 3 hours, storing milk at 4°C for 4 days to maintain supply.
1.4 Long-Term Lifestyle Modifications To Prevent Type 2 Diabetes
- Long-term lifestyle modifications post-GDM focus on sustained weight management targeting BMI <25 kg/m² through caloric deficit of 500 kcal/day via portion control and increased fiber >25 g/day to enhance satiety via delayed gastric emptying, reducing type 2 diabetes incidence by 50-60% over 10 years.
- Physical activity ≥150 min/week moderate aerobic like cycling activates peroxisome proliferator-activated receptor gamma improving adiponectin levels for insulin sensitization, combined with resistance training building muscle mass for glucose disposal.
- Dietary patterns emphasize Mediterranean style with monounsaturated fats >30% calories from olive oil, limiting refined sugars <25 g/day to prevent beta-cell exhaustion from glucotoxicity.
- Annual screening with 75 g OGTT detects prediabetes early, allowing metformin if lifestyle insufficient, with smoking cessation and alcohol <14 units/week further mitigating cardiovascular risks elevated 2-fold post-GDM.
- Weight loss mechanisms: 7-10% reduction improves hepatic insulin sensitivity, decreasing gluconeogenesis via reduced intrahepatic triglycerides.
- Exercise benefits: High-intensity interval training alternates bursts elevating catecholamines for lipolysis.
- Dietary specifics: Glycemic load <80/day from whole grains, vegetables to minimize postprandial spikes.
- Behavioral strategies: Self-efficacy building via goal-setting, tracking apps for accountability, family involvement for sustainability.
Nursing Insights
- Nurses tailor modifications by setting SMART goals like 30 min walks 5 days/week, following up quarterly to adjust for barriers like fatigue.
- In ethnic groups, nurses incorporate cultural foods low in glycemic index, teaching label reading to avoid hidden sugars.
Summary
- Diabetes in pregnancy, including preexisting type 1/2 diabetes mellitus and gestational diabetes mellitus (GDM), involves altered glucose metabolism from hormonal insulin resistance, requiring vigilant management to prevent maternal complications like preeclampsia/DKA and fetal issues such as macrosomia/anomalies.
- Key concepts: Pathophysiology highlights placental hormones like hPL inducing resistance; types differentiate chronic preexisting from transient GDM; risk factors include obesity/ethnicity; screening uses OGTT at 24-28 weeks; complications encompass maternal hypertension/polyhydramnios and fetal IUGR/RDS/hypoglycemia.
- Management strategies: Preconception A1c <6.5%, MNT with 40-50% carbs, exercise 150 min/week, insulin/oral agents, SMBG 4-7x/day, intrapartum glucose control, postpartum OGTT.
- Fetal surveillance: NST reactive with accelerations, BPP score ≥8/10, AFI 5-25 cm, ultrasound for growth >90th/<10th percentile.
- Patient education: SMBG techniques, hypo/hyperglycemia management (15-15 rule/insulin correction), breastfeeding for sensitivity, long-term lifestyle to prevent type 2 DM via weight/activity.
Naxlex
Videos
Login to View Video
Click here to loginTake Notes on Diabetes Mellitus
This filled cannot be empty
Join Naxlex Nursing for nursing questions & guides! Sign Up Now


