Use the table below to answer the question.
| Object | Mass | Time of Fall |
| | | |
|
| 1 |
| 5.0 g| 2.0 sec |
| |
|
| 2 |
| 5.0 g| 1.0 sec |
| |
|
| 3 |
| 30.0 g| 0.5 sec |
| |
|
| 4 |
| 35.0 g| 1.5 sec |
| |
Which of the following conclusions is supported by the data?
Objects A and B will fall at the same rate.
The greater the mass of an object, the faster it will fall.
The time of fall is independent of the mass of the object.
Air resistance is greater for A than for B.
The Correct Answer is C
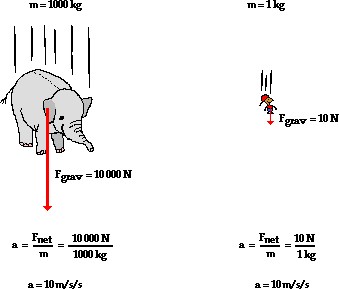
The data in the table does not support any of the conclusions a, b or d. The only conclusion that can be drawn from the data is c. The time of fall is independent of the mass of the object. This is because objects 1 and 2 have the same mass but different times of fall, while objects 3 and 4 have different masses but also different times of fall.
A. Objects A and B are not mentioned in the table, so this conclusion cannot be drawn from the data.
B. The data does not support the conclusion that the greater the mass of an object, the faster it will fall. In fact, object 3 has a greater mass than object 2 but falls in less time.
C. Air resistance is not mentioned in the table, so this conclusion cannot be drawn from the data.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
The correct answer is c. 100,000. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A pH 4 solution has a hydrogen-ion concentration that is 10^5 (or 100,000) times greater than that of a pH 9 solution.
A. 0.00001 is the hydrogen-ion concentration of a pH 9 solution as compared with a pH 4 solution.
B. 5 is the difference in pH units between a pH 4 solution and a pH 9 solution.
D. 50 is not the correct answer.

Correct Answer is A
Explanation
To accurately measure the density of a series of small irregular solids made of plastic, wood, fiberglass, and glass, a student will need a graduated cylinder, water, and a weighing balance. The student can use the water displacement method to determine the volume of each solid by measuring the volume of water displaced when the solid is submerged in a graduated cylinder filled with water. The mass of each solid can be measured using a weighing balance. The density can then be calculated by dividing the mass by the volume.

The other options are not correct because they do not provide the necessary tools to accurately measure the density of the solids. A spectrophotometer is used to measure light absorption and is not necessary for measuring density. A graduated beaker is less accurate than a graduated cylinder for measuring volume. A Bunsen burner is used for heating and is not necessary for measuring density.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
