Which of the following occurs in an oxidation reaction?
Removal of oxygen
Addition of carbon
Addition of neutrons
Removal of electrons
The Correct Answer is D
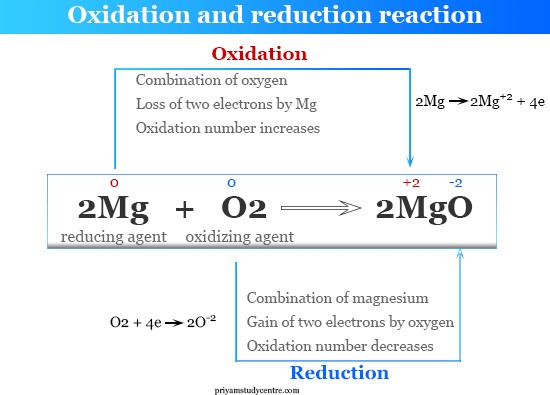
An oxidation reaction occurs when there is a removal of electrons. Oxidation is the loss of electrons during a reaction by a molecule, atom, or ion. When oxidation occurs, the oxidation state of the chemical species increases.
The other options are not correct because they do not accurately describe what occurs in an oxidation reaction. Removal of oxygen, addition of carbon, and addition of neutrons are not processes that occur in an oxidation reaction.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
It was frozen in the cold temperature of the Alps shortly after death and remained frozen until it was found. Freezing can preserve a body by slowing down or stopping the decomposition process.
Correct Answer is A
Explanation
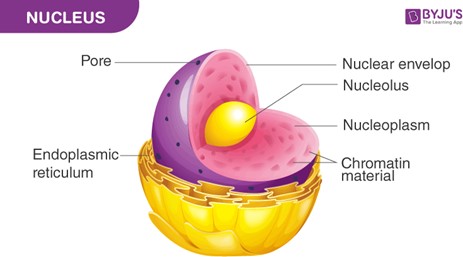
Nuclei. Genetic information describing the characteristics of an organism is found in the nuclei of its cells. The nucleus contains the organism's DNA, which carries the genetic information that determines its traits.
B. Membranes are structures that surround and enclose cells and organelles, but they do not contain genetic information.
C. Cilia are hair-like structures that protrude from the surface of some cells and are involved in movement, but they do not contain genetic information.
D. Ribosomes are organelles that are involved in protein synthesis, but they do not contain genetic information.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
