Which of the following best describes the result of using a catalyst in a chemical reaction?
The reaction is completed in a shorter amount of time.
A more desirable product is often formed.
A greater amount of heat energy is released by the reaction.
The yield of product is increased.
The Correct Answer is A
A catalyst is a substance that increases the rate of a chemical reaction without being consumed by the reaction.
As a result, the reaction is completed in a shorter amount of time.
Choice B is not correct because using a catalyst does not necessarily result in the formation of a more desirable product.
Choice C is not correct because using a catalyst does not necessarily result in the release of a greater amount of heat energy by the reaction.
Choice D is not correct because using a catalyst does not necessarily increase the yield of product.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
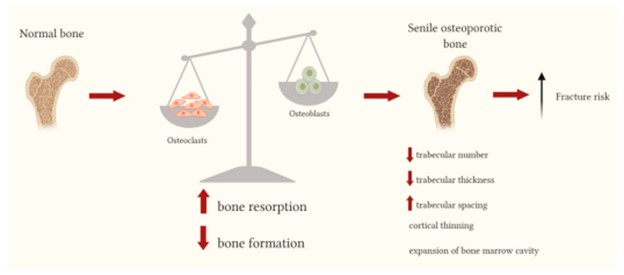
Osteoporosis is caused by an imbalance between the functioning of osteoclast and osteoblast cells.
Osteoblasts are responsible for forming new bone, while osteoclasts break down old bone.
If osteoblast activity declines while osteoclast activity continues at expected levels, this means that more bone is being broken down than is being formed, leading to a loss of bone density and an increased risk of osteoporosis.
Choice A is incorrect because an increase in osteocyte activity would not result in osteoporosis.
Osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Choice B is incorrect because a decline in osteoclast activity would not result in osteoporosis.
Osteoclasts break down old bone, so a decline in their activity would mean that less bone is being broken down.
Choice C is incorrect because an increase in osteocyte activity would not result in osteoporosis.
As mentioned earlier, osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Correct Answer is C
Explanation
It can also be caused by an underwater or coastal landslide, the eruption of a volcano, or the impact of a meteor or comet in a body of water.
Choice A is not correct because sunspot activity does not cause tsunamis.
Choice B is not correct because lightning strikes do not cause tsunamis. Choice D is not correct because flooding does not cause tsunamis.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
