A nurse is caring for a client who has the following arterial blood gas results: HCO3-, 18 mEq, PaCO, 28 mm Hg and pH 7.30. The nurse recognizes the client is experiencing which of the following acid base imbalances?
Respiratory acidosis
Metabolic alkalosis
Respiratory alkalosis
Metabolic acidosis
The Correct Answer is D
A. Respiratory acidosis would typically involve an elevated PaCO2, which is not seen in this case.
B. Metabolic alkalosis is characterized by an elevated bicarbonate level, which is not present in this scenario.
C. Respiratory alkalosis would present with a low PaCO2 and an elevated pH, which is not the case here.
D. The low bicarbonate level (HCO3) 18mEq/L (normal range of 22-26 mEq/L), and low pH 7.30 (normal range of 7.35-7.45), indicate metabolic acidosis. suggesting acidemia. The PaCO2 is also low at 28 mm Hg, indicating a respiratory compensation for the metabolic acidosis.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is ["A","B","C","D","E","F"]
Explanation
The nurse should report the hoarseness of the client's voice, which could indicate recurrent laryngeal nerve damage, which is a risk associated with thyroid surgery. Tingling around the mouth may suggest hypocalcemia, a common complication after thyroidectomy due to accidental removal or damage to the parathyroid glands. The moderate serosanguinous drainage on the neck dressing could signify bleeding or infection, which requires immediate attention. The noted tremor in both hands and the increase in temperature could be signs of a thyroid storm, a rare but life-threatening condition.
Furthermore, the client's restlessness could be a response to discomfort or could indicate a more serious issue, such as an impending thyrotoxic crisis. Oxygen saturation at 95% is within normal limits postoperatively; however, it should be monitored closely.
Correct Answer is D
Explanation
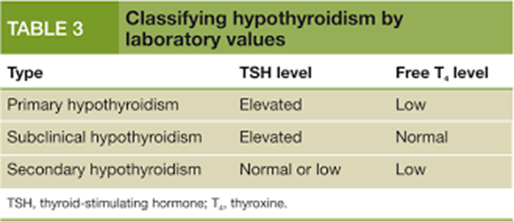
A. In primary hypothyroidism, the thyroid gland fails to produce sufficient thyroid hormone.
Consequently, free T4 levels are typically decreased.
B. Although serum T3 levels may also decrease in primary hypothyroidism due to impaired thyroid function, TSH is the primary marker used for diagnosis and monitoring.
C. Similarly, serum T4 levels may decrease in primary hypothyroidism due to decreased synthesis by the thyroid gland.
D. In primary hypothyroidism, the anterior pituitary gland releases more TSH to stimulate the thyroid gland to produce thyroid hormones. Therefore, elevated TSH levels are characteristic of primary hypothyroidism.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
