Which adverse effect unique to vancomycin is caused by histamine release?
Discoloration of body fluids
Ototoxicity
Red-man syndrome
Nephrotoxicity
The Correct Answer is C
A. Discoloration of body fluids:
Vancomycin can cause discoloration of body fluids, particularly urine, resulting in a brownish discoloration. However, this is not caused by histamine release.
B. Ototoxicity:
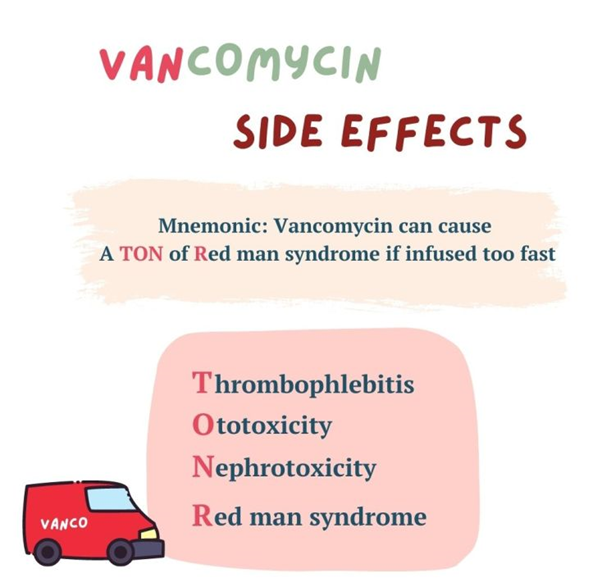
Ototoxicity refers to damage to the inner ear structures leading to hearing loss or balance problems. While vancomycin can cause ototoxicity, it is not specifically associated with histamine release.
C. Red-man syndrome
Red-man syndrome, also known as red-neck syndrome or red-person syndrome, is a hypersensitivity reaction characterized by flushing of the skin, particularly the upper body and face, resembling a "red man." This reaction is typically associated with the rapid infusion of vancomycin and is caused by the release of histamine from mast cells and basophils. It is not an allergic reaction but rather a non-immunologic response to vancomycin.
D. Nephrotoxicity:
Nephrotoxicity refers to kidney damage caused by certain medications or toxins. While vancomycin can cause nephrotoxicity, it is not specifically associated with histamine release.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
A. Never take with prescription medications:
This statement is not accurate. Many herbal products can interact with prescription medications, potentially leading to adverse effects or reduced efficacy. However, avoiding taking herbal products with prescription medications altogether may not be practical or necessary in all cases. Instead, it's essential to assess for potential interactions and consult with healthcare professionals.
B. Use only one herbal preparation at a time:
This is the correct approach. Using only one herbal preparation at a time allows for better identification of any allergic reactions or adverse effects. If multiple herbal products are taken simultaneously, it can be challenging to determine which product is causing a particular reaction. Starting with one product also simplifies monitoring for efficacy and safety.
C. Take less than the recommended dose initially:
While starting with a lower dose initially may be a prudent approach for some individuals, it is not necessarily the best way to identify allergic or adverse reactions. Taking less than the recommended dose may not provide a full assessment of the product's effects and may not adequately identify potential adverse reactions.
D. Check with a reputable pharmacist:
Consulting with a reputable pharmacist is essential for obtaining information about herbal products, including potential interactions, side effects, and recommended dosages. While a pharmacist can provide valuable guidance, they may not be present during the initial use of the herbal product to monitor for adverse reactions directly.
Correct Answer is C
Explanation
A. Echinacea:
Echinacea is commonly used to support the immune system and may be used to prevent or reduce the severity of colds and other infections. However, it is not typically used specifically for treating menopause-related hot flashes.
B. Saw palmetto:
Saw palmetto is primarily used for managing symptoms related to the prostate gland, such as benign prostatic hyperplasia (BPH), and is not commonly used for treating menopause-related hot flashes in women.
C. Black cohosh:
Black cohosh is one of the most widely studied herbal remedies for managing menopause-related symptoms, including hot flashes. Research suggests that black cohosh may help reduce the frequency and severity of hot flashes in some women experiencing menopausal symptoms.
D. Cranberry juice:
Cranberry juice is often used to promote urinary tract health and prevent urinary tract infections (UTIs). While it may have some health benefits, cranberry juice is not typically used for managing menopause-related hot flashes.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
