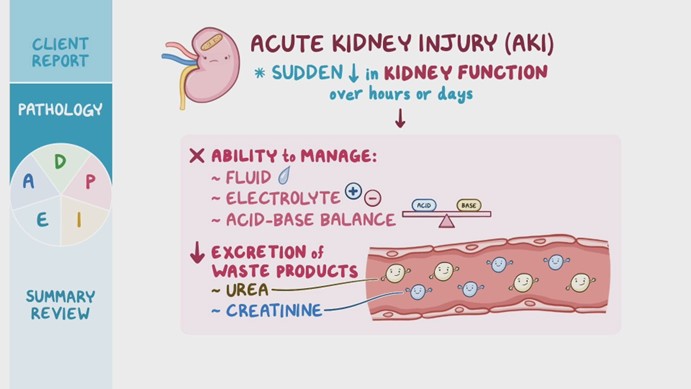
A nurse is caring for a client who has acute kidney injury (AKI). Which of the following arterial blood gas values would the nurse expect this client to have?
pH 7.49, HCO3 24, PaCO2 30
pH 7.26, HCO3 24, PaCO2 46
pH 7.26, HCO3 14, PaCO2 30
pH 7.49, HCO3 30, PaCO2 40
The Correct Answer is C
Choice A Reason: This choice is incorrect because it indicates respiratory alkalosis, not AKI. Respiratory alkalosis is a condition in which the lungs eliminate too much carbon dioxide (CO2) from the blood, resulting in a low level of CO2 (PaCO2) and a high level of pH. A normal PaCO2 range is 35 to 45 mm Hg, and a normal pH range is 7.35 to 7.45, so a value of 30 mm Hg and 7.49 indicate respiratory alkalosis.
Choice B Reason: This choice is incorrect because it indicates respiratory acidosis, not AKI. Respiratory acidosis is a condition in which the lungs cannot eliminate enough CO2 from the blood, resulting in a high level of CO2 (PaCO2) and a low level of pH. A value of 46 mm Hg and 7.26 indicate respiratory acidosis.
Choice C Reason: This choice is correct because it indicates metabolic acidosis, which is a common complication of AKI. Metabolic acidosis is a condition in which the body produces too much acid or loses too much base, resulting in a low level of bicarbonate (HCO3) and a low level of pH. A normal HCO3 range is 22 to 26 mEq/L, so a value of 14 mEq/L indicates metabolic acidosis. The low PaCO2 value (30 mm Hg) is due to the respiratory compensation mechanism that tries to restore the acid-base balance by increasing the ventilation and eliminating more CO2.
Choice D Reason: This choice is incorrect because it indicates metabolic alkalosis, not AKI. Metabolic alkalosis is a condition in which the body loses too much acid or gains too much base, resulting in a high level of bicarbonate (HCO3) and a high level of pH. A value of 30 mEq/L and 7.49 indicate metabolic alkalosis.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
Choice A Reason: This is incorrect because hypervolemia is a condition of excess fluid volume in the body. A client who has an extensive burn injury is more likely to have hypovolemia, which is a condition of low fluid volume, due to fluid loss from the damaged skin and capillaries.
Choice B Reason: This is incorrect because metabolic alkalosis is a condition of high blood pH and high bicarbonate level. A client who has an extensive burn injury is more likely to have metabolic acidosis, which is a condition of low blood pH and low bicarbonate level, due to increased production of lactic acid and ketones from tissue hypoxia and breakdown.
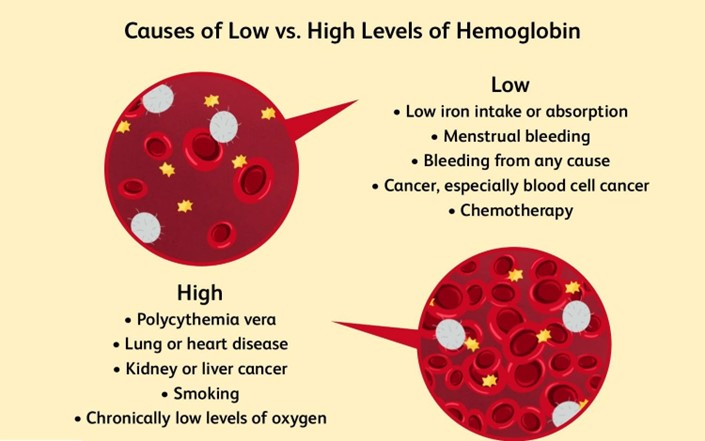
Choice C Reason: This is correct because low hemoglobin is a common laboratory finding in a client who has an extensive burn injury. Hemoglobin is the protein in red blood cells that carries oxygen. A client who has an extensive burn injury may have low hemoglobin due to hemolysis, which is the destruction of red blood cells, or hemorrhage, which is the loss of blood.
Choice D Reason: This is incorrect because hyperkalemia is a condition of high blood potassium level. A client who has an extensive burn injury may have hyperkalemia in the early phase of injury, due to cell damage and potassium release, but it is usually transient and followed by hypokalemia, which is a condition of low blood potassium level, due to fluid loss and potassium depletion.
Correct Answer is ["125"]
Explanation
Step 1: Determine the total time required to infuse 40 mEq at a rate of 10 mEq/hr.
40 mEq ÷ 10 mEq/hr = 4 hours
Result: 4 hours
Step 2: Determine the infusion rate in mL/hr.
500 mL ÷ 4 hours = 125 mL/hr
Result: 125 mL/hr
The nurse should set the IV pump to deliver 125 mL/hr.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
