A client is receiving intravenous fluid therapy with 0.9% sodium chloride solution.
The nurse understands that this type of solution is classified as:
Hypotonic.
Isotonic.
Hypertonic.
Colloid.
Colloid.
The Correct Answer is B
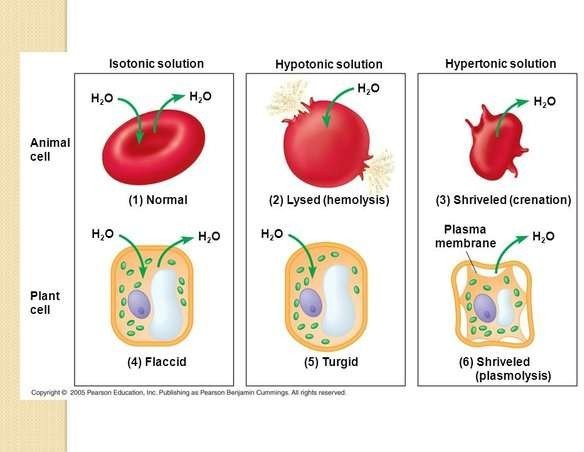
An isotonic solution has the same concentration of solutes as the blood plasma. 0.9% sodium chloride solution is an example of an isotonic solution.
It is used to supply water and salt to the body and to prevent hypotension induced by spinal anaesthesia.
Choice A is wrong because a hypotonic solution has a lower concentration of solutes than the blood plasma.
It can cause water to move into the cells and cause them to swell.
Choice C is wrong because a hypertonic solution has a higher concentration of solutes than the blood plasma.
It can cause water to move out of the cells and cause them to shrink.
Choice D is wrong because a colloid solution contains large molecules that do not pass through the capillary walls.
It is used to increase the blood volume and pressure in cases of shock or severe blood loss.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Performing hand hygiene before and after handling the dialysis equipment is essential to prevent infection in peritoneal dialysis.

Hand washing and appropriate use of a mask can help avoid peritonitis, which is a serious complication of peritoneal dialysis.
Choice A is wrong because administering antibiotics prophylactically is not recommended for peritoneal dialysis patients, as it can increase the risk of antibiotic resistance and adverse effects.
Choice C is wrong because allowing the client to handle the dialysis equipment independently may increase the risk of contamination and infection.
The client should be supervised and instructed by a nurse on how to use sterile technique when connecting and disconnecting the transfer set.
Choice D is wrong because discontinuing the peritoneal dialysis if the client develops a fever may worsen the client’s condition and lead to fluid overload and electrolyte imbalance.
The client should be evaluated for signs of infection and treated accordingly.
Correct Answer is ["A","C","D"]
Explanation
Furosemide is a loop diuretic that causes the kidneys to excrete more water and salt, which can lead to dehydration and electrolyte imbalance.

Electrolyte imbalance can cause muscle cramps, numbness and tingling, weakness and fatigue, and other symptoms.
Therefore, the client should monitor for these signs and report them to the doctor if they occur.
Choice B is wrong because dry mouth is not a sign of electrolyte imbalance, but rather a sign of dehydration.
Dehydration can also cause thirst, decreased urination, drowsiness, and confusion.
Choice E is wrong because tachycardia is not a sign of electrolyte imbalance, but rather a sign of hypovolemia (low blood volume) or hypotension (low blood pressure).
Furosemide can lower blood pressure by reducing fluid volume in the body.
Therefore, the client should also monitor their blood pressure and pulse regularly while taking furosemide.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
