Which of the following growth curves shows a population that is at its carrying capacity?
B
C
A
D
The Correct Answer is A
A population is said to be at its carrying capacity when it has reached the maximum number of individuals that can be sustained in a particular environment over a prolonged period of time, given the available resources and the prevailing environmental conditions.
In other words, carrying capacity refers to the maximum population size that a given ecosystem can support without being depleted of resources or experiencing environmental degradation. Once a population reaches its carrying capacity, its growth rate slows down and stabilizes, as individuals start to compete more intensely for resources such as food, water, and shelter, and mortality rates increase.
Carrying capacity is an important concept in ecology and population biology because it helps to explain the dynamics of natural populations and how they are influenced by changes in the environment, such as climate change, habitat loss, and human activities.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
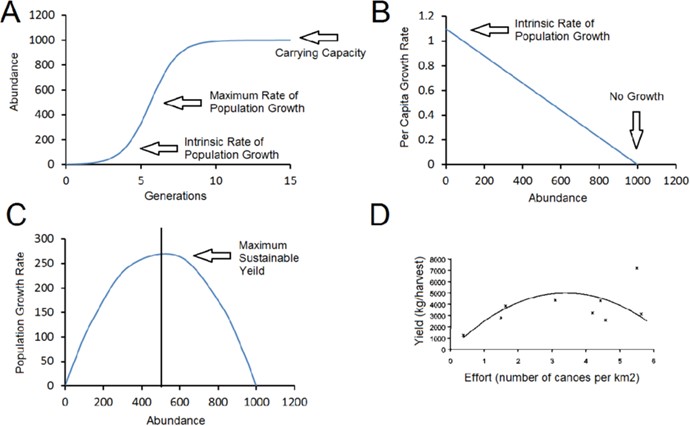
Correct Answer is D
Explanation
An oxidation reaction occurs when there is a removal of electrons. Oxidation is the loss of electrons during a reaction by a molecule, atom, or ion. When oxidation occurs, the oxidation state of the chemical species increases.
The other options are not correct because they do not accurately describe what occurs in an oxidation reaction. Removal of oxygen, addition of carbon, and addition of neutrons are not processes that occur in an oxidation reaction.
Correct Answer is C
Explanation
The correct answer is c. 100,000. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A pH 4 solution has a hydrogen-ion concentration that is 10^5 (or 100,000) times greater than that of a pH 9 solution.
A. 0.00001 is the hydrogen-ion concentration of a pH 9 solution as compared with a pH 4 solution.
B. 5 is the difference in pH units between a pH 4 solution and a pH 9 solution.
D. 50 is not the correct answer.

Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
