To accurately measure the density of a series of small irregular solids made of plastic, wood, fiberglass, and glass, a student will need which of the following laboratory tools?
Graduated cylinder, water, weighing balance
Graduated cylinder, spectrophotometer, water
Graduated beaker, metric ruler, water
Weighing balance, Bunsen burner, metric ruler
The Correct Answer is A
To accurately measure the density of a series of small irregular solids made of plastic, wood, fiberglass, and glass, a student will need a graduated cylinder, water, and a weighing balance. The student can use the water displacement method to determine the volume of each solid by measuring the volume of water displaced when the solid is submerged in a graduated cylinder filled with water. The mass of each solid can be measured using a weighing balance. The density can then be calculated by dividing the mass by the volume.

The other options are not correct because they do not provide the necessary tools to accurately measure the density of the solids. A spectrophotometer is used to measure light absorption and is not necessary for measuring density. A graduated beaker is less accurate than a graduated cylinder for measuring volume. A Bunsen burner is used for heating and is not necessary for measuring density.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
The unknown genotype is Aa. This can be inferred from the Punnet square, which shows that half of the offspring are Aa and half are aa. This indicates that the unknown parent must have one dominant allele (A) and one recessive allele (a), making its genotype Aa.
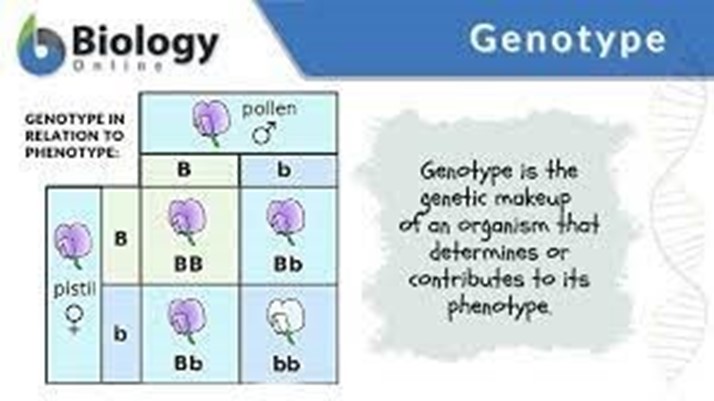
The other options are not correct because they do not match the results shown in the Punnet square. If the unknown genotype was aa or AA, all of the offspring would have the same genotype as their parent. If the unknown genotype was a, it would not be a valid genotype as it only has one allele.
Correct Answer is D
Explanation
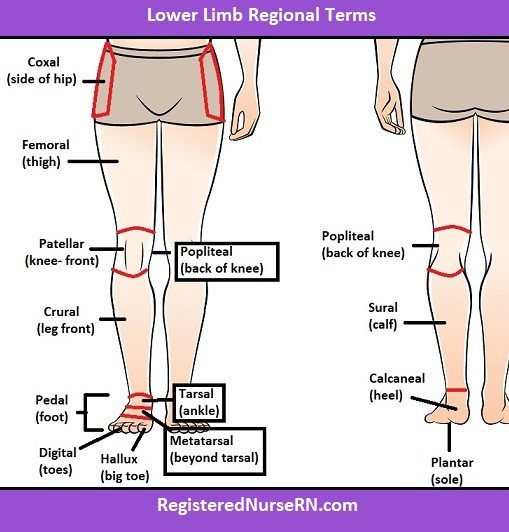
The tibia and fibula are located in the crural region of the body, which is the lower leg between the knee and ankle. The coxal region refers to the hip area, the antecubital region is the front of the elbow, and the tarsal region is the ankle and foot
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
