How does the use of a catalyst affect the activation energy of a chemical reaction?
It increases the activation energy required for the reaction.
It decreases the activation energy required for the reaction.
It has no effect on the activation energy required for the reaction.
It increases the rate of reaction but has no effect on the activation energy.
The Correct Answer is B
A catalyst provides a new reaction pathway in which a lower activation energy is offered.
This allows more reactant molecules to collide with enough energy to surmount the smaller energy barrier, increasing the rate of reaction 2.
Choice A, It increases the activation energy required for the reaction, is not the correct answer because it describes the opposite effect of a catalyst.
Choice C, It has no effect on the activation energy required for the reaction, is not the correct answer because a catalyst does have an effect on activation energy.
Choice D, It increases the rate of reaction but has no effect on the activation energy, is not the correct answer because a catalyst increases the rate of reaction by decreasing the activation energy.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
Inflammatory cytokines released during the early response to bacterial infection play a crucial role in initiating cell recruitment and local inflammation 1.
They induce the expression of adhesion molecules in endothelial cells and promote the recruitment of neutrophils to the site of inflammation 1.
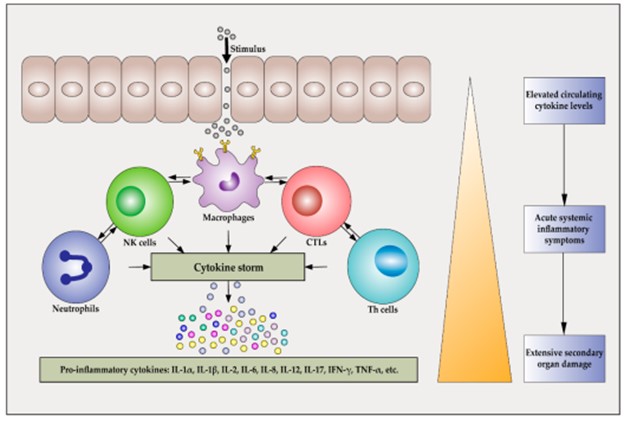
Choice A is incorrect because while inflammatory cytokines may enhance phagocytosis, they do not directly disrupt the infection.
Choice B is incorrect because inflammatory cytokines do not directly attack invading pathogens.
Choice D is incorrect because inflammatory cytokines do not secrete antibodies to neutralize pathogens.
Correct Answer is A
Explanation
The neuromuscular junction is a type of synapse where neuronal signals from the brain or spinal cord interact with skeletal muscle fibers, causing them to contract.
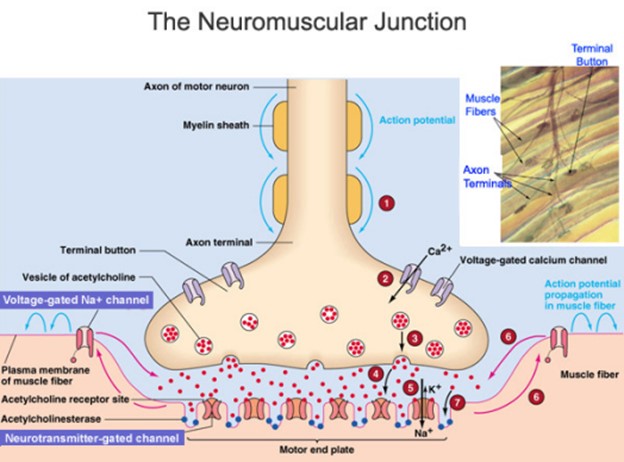
The activation of many muscle fibers together causes muscles to contract, which in turn can produce movement.
Choice B is incorrect because binding acetylcholine to nAChRs is a process that occurs at the neuromuscular junction, but it is not the function of the neuromuscular junction itself.
Choice C is incorrect because depolarizing the muscle cell membrane is a result of the function of the neuromuscular junction, but it is not the function itself.
Choice D is incorrect because activating voltage-gated sodium channels on the muscle membrane is a result of the function of the neuromuscular junction, but it is not the function itself.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
