What is the relationship between atomic mass and mass number?
They are the same.
Atomic mass is always greater than mass number.
Atomic mass and mass number are not related.
Atomic mass is very close to mass number but with some deviation in the decimal places.
The Correct Answer is D
Atomic mass is very close to mass number but with some deviation in the decimal places.
Atomic mass is also known as atomic weight and is the weighted average mass of an atom of an element based on the relative natural abundance of that element’s isotopes.
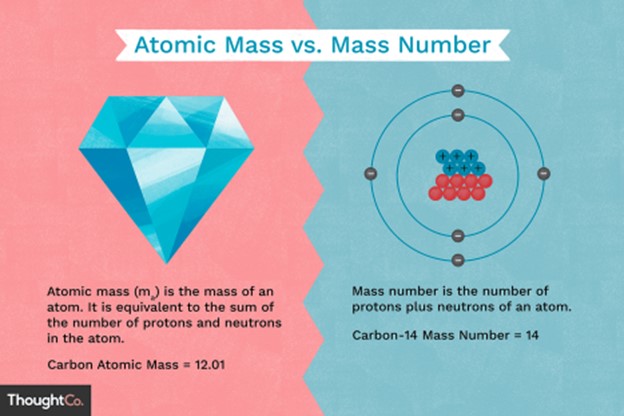
The mass number, on the other hand, is a count of the total number of protons and neutrons in an atom’s nucleus.
Choice A is incorrect because atomic mass and mass number do not mean the same thing.
Choice B is incorrect because atomic mass is not always greater than mass number.
Choice C is incorrect because atomic mass and mass number are related.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration.
The concentration of solute particles in the solution is the primary factor that determines the movement of water across cell membranes in osmosis.
Hydrostatic pressure (choice A) can affect the movement of water across cell membranes but is not the primary factor responsible for osmosis.
Temperature (choice C) can affect the rate of osmosis but is not the primary factor responsible for osmosis.
Kinetic energy of liquid water molecules (choice D) can affect the rate of osmosis but is not the primary factor responsible for osmosis.
Correct Answer is A
Explanation
An increase in viscosity of a fluid results in a decrease in mobility of particles.
Viscosity is the resistance of a fluid to a change in shape or movement of neighbouring portions relative to one another.
It denotes opposition to flow and may be thought of as internal friction between the molecules.
Choice B is incorrect because an increase in viscosity does not affect the density of a fluid.
Choice C is incorrect because an increase in viscosity results in a decrease, not an increase, in flow rate.
Choice D is incorrect because an increase in viscosity does not affect the pressure of a fluid.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
