Which factor is primarily responsible for the movement of water across cell membranes in osmosis?
Hydrostatic pressure of the solution.
Concentration of solute particles in the solution.
Temperature of the solution.
Kinetic energy of liquid water molecules .
The Correct Answer is B
Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration.
The concentration of solute particles in the solution is the primary factor that determines the movement of water across cell membranes in osmosis.
Hydrostatic pressure (choice A) can affect the movement of water across cell membranes but is not the primary factor responsible for osmosis.
Temperature (choice C) can affect the rate of osmosis but is not the primary factor responsible for osmosis.
Kinetic energy of liquid water molecules (choice D) can affect the rate of osmosis but is not the primary factor responsible for osmosis.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
The plasma membrane is the organelle that encapsulates the contents of the cell and plays a vital role in regulating the movement of substances in and out of the cell.
It is a selectively permeable barrier that separates the internal environment of the cell from the external environment.
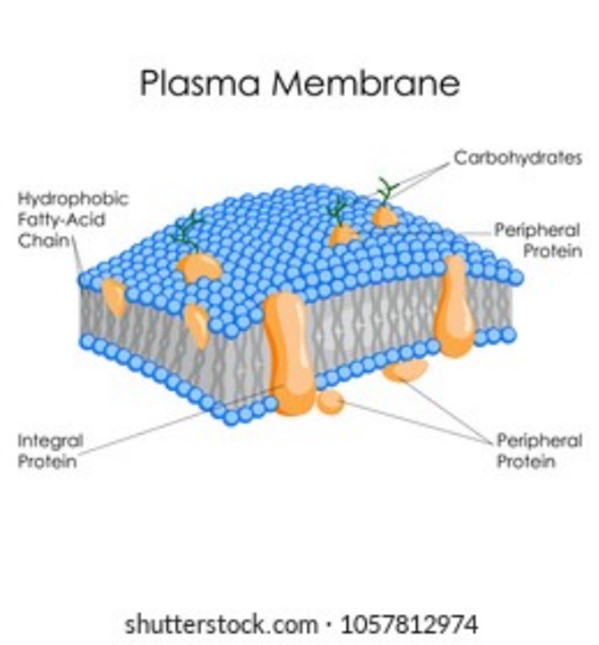
Choice A is incorrect because ribosomes are organelles involved in protein synthesis, not in regulating the movement of substances in and out of the cell.
Choice B is incorrect because the nucleus is an organelle that contains the cell’s genetic material, not in regulating the movement of substances in and out of the cell.
Choice C is incorrect because mitochondria are organelles involved in energy production, not in regulating the movement of substances in and out of the cell.
Correct Answer is D
Explanation
The medication prescribed to the patient is a diuretic, which removes water and electrolytes from the body by increasing urination 1.
This helps reduce fluid buildup in the body.
Choice A, Inhibits the renin-angiotensin-aldosterone system, is not the correct answer because it describes a different mechanism of action.
Choice B, Blocks beta receptors, is not the correct answer because it describes a different mechanism of action.
Choice C, Increases sodium and water reabsorption, is not the correct answer because it would have the opposite effect of reducing fluid buildup.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
