What is a control group used for in scientific studies?
To establish causality by isolating the effect of an independent variable.
To establish the effect of a dependent variable on an independent variable.
To control the impact of extraneous variables on the dependent variable.
To control the impact of extraneous variables on the independent variable.
The Correct Answer is A
A control group is used in scientific studies to establish causality by isolating the effect of an independent variable.
The control group serves as a baseline or comparison group that does not receive the treatment or intervention being tested.
By comparing the results of the control group to the experimental group, researchers can determine if any observed changes are due to the independent variable or if they are due to chance or other factors.
Choice B is incorrect because a control group is not used to establish the effect of a dependent variable on an independent variable.
Choice C is incorrect because while a control group can help control for the impact of extraneous variables on the dependent variable, its primary purpose is to isolate the effect of the independent variable.
Choice D is incorrect because a control group is not used to control for the impact of extraneous variables on the independent variable.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
Granzymes are a family of serine proteases that are stored in and secreted from the cytotoxic granules of cytotoxic T lymphocytes (CTL) and natural killer (NK) cells.
They work in synergy with perforin, a pore-forming toxin, to induce apoptosis in target cells.
Perforin is necessary for the delivery of granzyme B to the target cell cytosol where caspase-dependent and -independent pathways to apoptosis are activated.
Perforins (choice A) are pore-forming toxins that work in synergy with granzymes to induce apoptosis in target cells.
Cytokines (choice B) are signaling molecules that regulate immune responses but do not directly induce apoptosis in target cells.
Interferons (choice D) are a type of cytokine that play a role in immune responses but do not directly induce apoptosis in target cells.
Correct Answer is B
Explanation
Glycogen is the storage form of glucose in the human body.
It is a polysaccharide that is stored primarily in the liver and muscle tissue and can be broken down into glucose when the body needs energy.
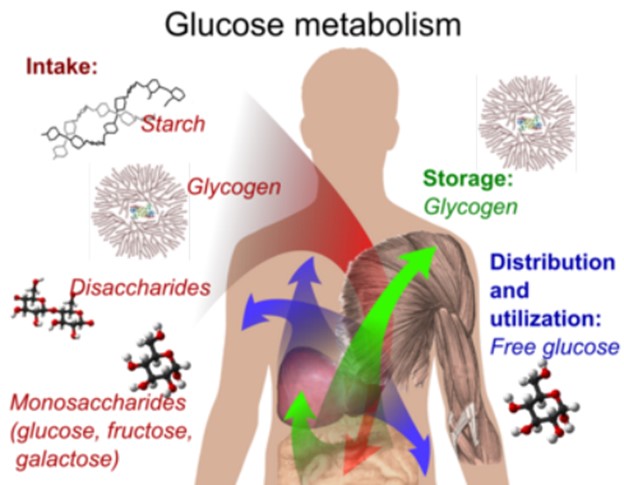
Choice A is incorrect because starch is a storage form of glucose in plants, not in the human body.
Choice C is incorrect because fructose is a simple sugar, not a storage form of glucose.
Choice D is incorrect because cellulose is a structural carbohydrate found in plant cell walls, not a storage form of glucose in the human body.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
