Experiments cannot validate hypotheses, only falsify them. The statement above can be restated in which of the following ways?
Until disproved, an explanation for an observation is valid.
Certain concepts cannot be subjected to direct experimentation.
A hypothesis that has not been falsified remains provisional.
Proving a hypothesis exempts it from further testing.
The Correct Answer is C
The statement "Experiments cannot validate hypotheses, only falsify them" can be restated as "A hypothesis that has not been falsified remains provisional." This means that a hypothesis is considered valid until it is disproved by experimental evidence. However, even if a hypothesis has not been falsified, it is still considered provisional and subject to further testing and scrutiny.
A. "Until disproved, an explanation for an observation is valid" is similar to the correct answer but does not capture the provisional nature of a hypothesis.
B. "Certain concepts cannot be subjected to direct experimentation" is not a restatement of the original statement.
D. "Proving a hypothesis exempts it from further testing" is incorrect because no hypothesis can be definitively proven and all hypotheses are subject to further testing and scrutiny.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4. The pH scale is a logarithmic scale, which means that each change of one pH unit represents a tenfold change in the hydrogen-ion concentration. A substance with a pH of 3 has a hydrogen-ion concentration that is 10 times greater than that of a substance with a pH of 4.
A. A substance with a pH of 3 is not two times more alkaline than a substance with a pH of 4.
B. A substance with a pH of 3 is not 10 times more alkaline than a substance with a pH of 4.
C. A substance with a pH of 3 is not two times more acidic than a substance with a pH of 4.
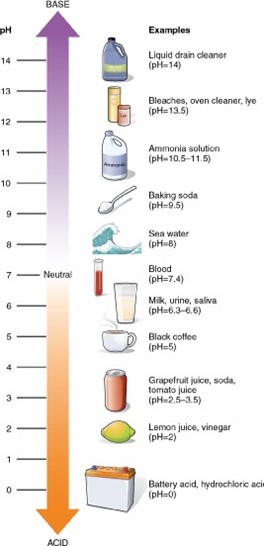
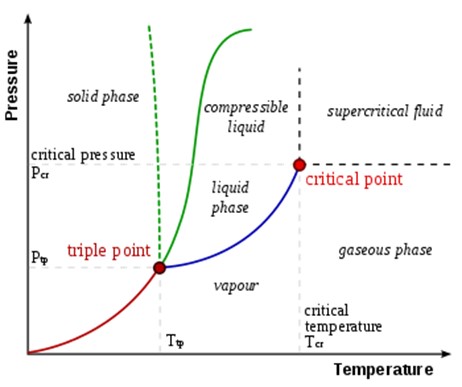
Correct Answer is D
Explanation
The triple point of a substance is the temperature and pressure at which the substance exists simultaneously in solid, liquid, and gas phases ¹. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium ¹.
The other options are not correct because they do not accurately describe the triple point of a substance. Sol, gel, and plasma are not phases that coexist at the triple
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
