Which of the following structures is present in both prokaryotic and eukaryotic cells?
Cell membrane.
Golgi apparatus
Chloroplast
Endoplasmic reticulum.
The Correct Answer is A
The cell membrane is a thin, flexible barrier that surrounds all cells and separates the inside of the cell from the outside environment.
It is composed of a lipid bilayer and regulates the movement of substances into and out of the cell.
Choice B is incorrect because the Golgi apparatus is not present in prokaryotic cells.
The Golgi apparatus is an organelle found in eukaryotic cells that is involved in modifying, sorting, and packaging proteins and lipids for transport to other parts of the cell or to be secreted outside the cell.
Choice C is incorrect because chloroplasts are not present in prokaryotic cells.
Chloroplasts are organelles found in plant cells and some algae that are responsible for photosynthesis.
Choice D is incorrect because the endoplasmic reticulum is not present in prokaryotic cells.
The endoplasmic reticulum is an organelle found in eukaryotic cells that is involved in protein synthesis and lipid metabolism.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
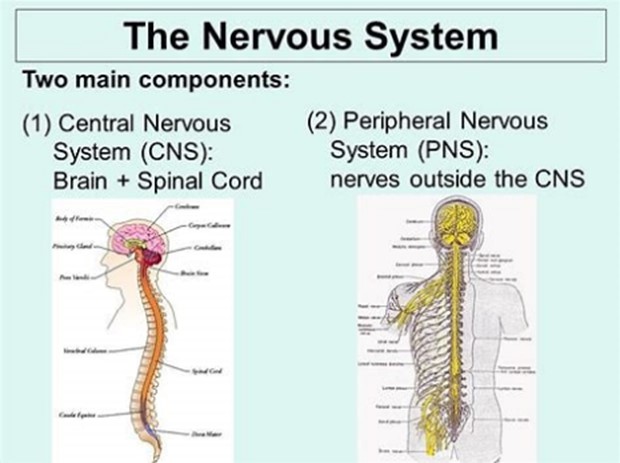
The two major parts of the nervous system are the Central Nervous System (CNS) and the Peripheral Nervous System (PNS).
The CNS comprises the brain and spinal cord and acts as the integration and command centre of the body.
The PNS represents the conduit between the CNS and the body and is further subdivided into the somatic nervous system (SNS) and the autonomic nervous system (ANS).
Choice A is incorrect because it only mentions two subdivisions of the PNS: the autonomic nervous system (ANS) and the somatic nervous system (SNS).
Choice B is incorrect because it only mentions one major part of the nervous system, the PNS, and one subdivision of it, the SNS.
Choice D is incorrect because it only mentions one major part of the nervous system, the CNS, and one subdivision of the PNS, the ANS.
Correct Answer is D
Explanation
The pulmonary veins are the vessels that carry oxygenated blood from the lungs to the left atrium of the heart.
Choice A is not correct because the superior vena cava carries deoxygenated blood from the upper body to the right atrium of the heart.
Choice B is not correct because the inferior vena cava carries deoxygenated blood from the lower body to the right atrium of the heart.
Choice C is not correct because the pulmonary artery carries deoxygenated blood from the right ventricle of the heart to the lungs.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
