Which of the following organic molecules contain both an amine and carboxyl group?
Lipids.
Chitin.
Cellulose.
Proteins
The Correct Answer is D
Proteins are made up of amino acids which are organic molecules that contain both an amine functional group (–NH2) and a carboxylic acid functional group (– COOH)1.
Choice A, Lipids, is not the correct answer because lipids are a group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, phospholipids, and others.
They do not contain both an amine and carboxyl group.
Choice B, Chitin, is not the correct answer because chitin is a long-chain polymer of N-acetylglucosamine, a derivative of glucose.
It does not contain both an amine and carboxyl group.
Choice C, Cellulose, is not the correct answer because cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.
It does not contain both an amine and carboxyl group.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
Nitrogen gas (N2) is an extremely stable molecule because it consists of two nitrogen atoms bonded together by a triple covalent bond.
A covalent bond is a type of chemical bond where atoms share electrons to form a molecule.
In a triple covalent bond, three pairs of electrons are shared between the two atoms, resulting in a very strong bond that makes the molecule extremely stable.
Choice A. Ionic bonds is not correct because ionic bonds involve the transfer of electrons from one atom to another to form ions, which are then attracted to each other due to their opposite charges.
Nitrogen gas does not contain ions and is not held together by ionic bonds.
Choice B. Hydrogen bonds is not correct because hydrogen bonds are weak electrostatic attractions between molecules that contain hydrogen atoms bonded to highly electronegative atoms such as oxygen or nitrogen.
Nitrogen gas does not contain hydrogen atoms and is not held together by hydrogen bonds.
Choice C. Resonance bonds is not correct because resonance refers to the delocalization of electrons in a molecule where multiple Lewis structures can be drawn to represent the molecule.
Nitrogen gas has a single Lewis structure and does not exhibit resonance.
Correct Answer is D
Explanation
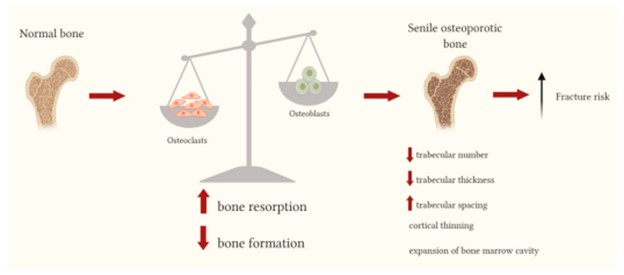
Osteoporosis is caused by an imbalance between the functioning of osteoclast and osteoblast cells.
Osteoblasts are responsible for forming new bone, while osteoclasts break down old bone.
If osteoblast activity declines while osteoclast activity continues at expected levels, this means that more bone is being broken down than is being formed, leading to a loss of bone density and an increased risk of osteoporosis.
Choice A is incorrect because an increase in osteocyte activity would not result in osteoporosis.
Osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Choice B is incorrect because a decline in osteoclast activity would not result in osteoporosis.
Osteoclasts break down old bone, so a decline in their activity would mean that less bone is being broken down.
Choice C is incorrect because an increase in osteocyte activity would not result in osteoporosis.
As mentioned earlier, osteocytes are mature bone cells that maintain the mineral concentration of the bone matrix.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
