A myocardial infarction affects which of the following blood vessels of the heart?
Coronary
Aorta
Pulmonary
Vena cava.
The Correct Answer is A
A myocardial infarction, commonly known as a heart attack, occurs when blood flow decreases or stops in the coronary artery of the heart, causing damage to the heart muscle.
Choice B is incorrect because the aorta is not a blood vessel of the heart.
The aorta is the main artery that carries oxygenated blood from the heart to the rest of the body.
Choice C is incorrect because the pulmonary blood vessels are not affected by a myocardial infarction.
The pulmonary blood vessels carry deoxygenated blood from the heart to the lungs.
Choice D is incorrect because the vena cava is not a blood vessel of the heart.
The vena cava is a large vein that carries deoxygenated blood from the body to the heart.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
As a solid turns to a liquid, the particles become less ordered and more free to move around.
Choice B is not correct because particles have an increase in mobility as a solid turns to a liquid.
Choice C is not correct because particles move further apart as a solid turns to a liquid.
Choice D is not correct because intermolecular forces between particles become weaker as a solid turns to a liquid.
Correct Answer is C
Explanation
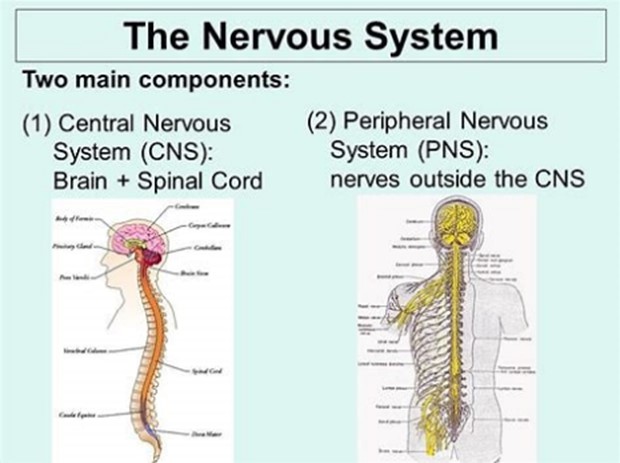
The two major parts of the nervous system are the Central Nervous System (CNS) and the Peripheral Nervous System (PNS).
The CNS comprises the brain and spinal cord and acts as the integration and command centre of the body.
The PNS represents the conduit between the CNS and the body and is further subdivided into the somatic nervous system (SNS) and the autonomic nervous system (ANS).
Choice A is incorrect because it only mentions two subdivisions of the PNS: the autonomic nervous system (ANS) and the somatic nervous system (SNS).
Choice B is incorrect because it only mentions one major part of the nervous system, the PNS, and one subdivision of it, the SNS.
Choice D is incorrect because it only mentions one major part of the nervous system, the CNS, and one subdivision of the PNS, the ANS.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
