Which of the following chemicals is released by one type of immune cell to directly activate another type of immune cell?
Cytokines
Lysozymes
Perforin
Granzymes.
The Correct Answer is A
Cytokines are chemicals released by one type of immune cell to directly activate another type of immune cell.
They are small proteins that act as messengers between cells and play a key role in regulating the immune response.
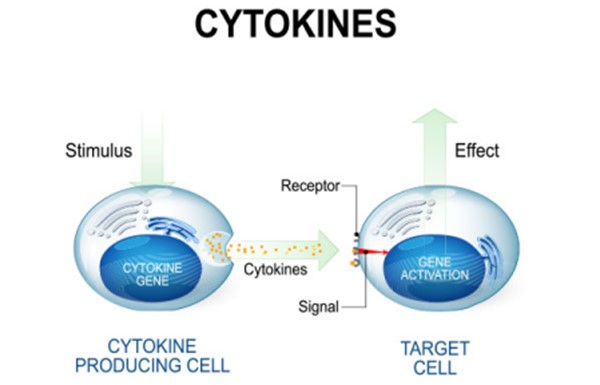
Choice B. Lysozymes is not the correct answer because lysozymes are enzymes that break down bacterial cell walls and do not directly activate other immune cells.
Choice C. Perforin is not the correct answer because perforin is a protein that forms pores in the membranes of target cells and does not directly activate other immune cells.
Choice D. Granzymes is not the correct answer because granzymes are enzymes that enter target cells and trigger apoptosis (cell death) and do not directly activate other immune cells.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
Genes are segments of DNA that encode the information for making proteins.
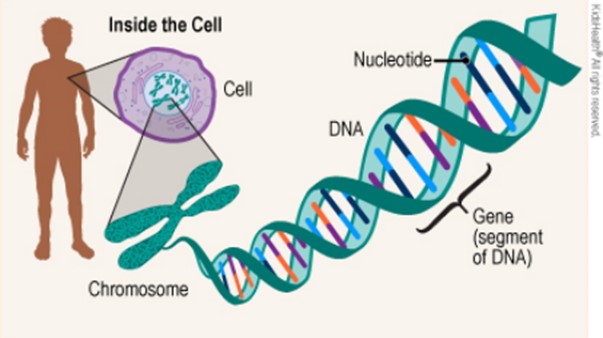
The sequence of nucleotides (As, Ts, Cs, and Gs) in a gene determines the amino acid sequence of the protein. DNA sequencing is the process of determining the sequence of nucleotides in a piece of DNA.
Choice A is incorrect because enzymes are proteins that catalyze chemical reactions, not DNA sequences. Choice B is incorrect because blood types are determined by the presence or absence of certain antigens on the surface of red blood cells, not by DNA sequences. Choice C is incorrect because hormones are chemical messengers that regulate various body functions, not DNA sequences.
Correct Answer is B
Explanation
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape. This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes cross-bridge formation, and muscle contraction begins.
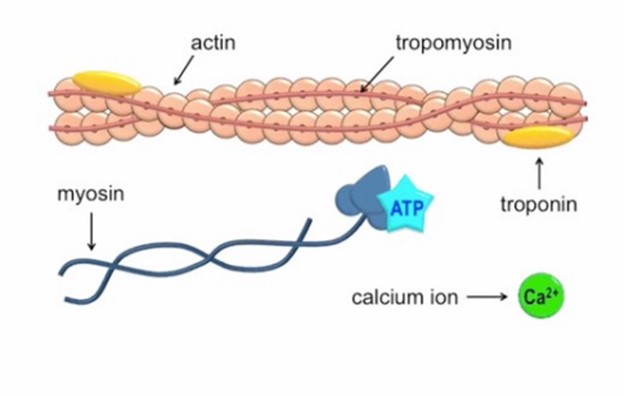
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism but do not bind to the troponin complex.
Sodium ions are important for generating action potentials but do not bind to the troponin complex.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
