Lithium has an atomic number of 3 and a mass number of 7.
Which of the following is the number of protons in a lithium atom?
7.
3.
12.
4.
The Correct Answer is B
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.
Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
Genes are segments of DNA that encode the information for making proteins.
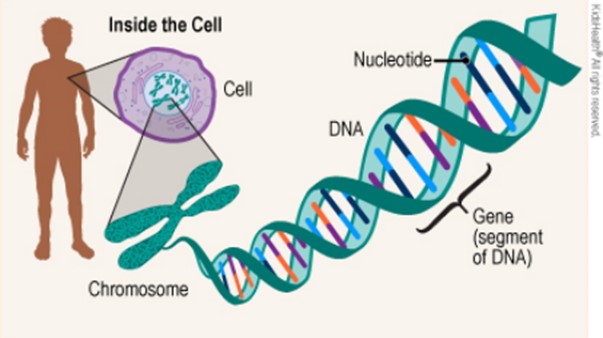
The sequence of nucleotides (As, Ts, Cs, and Gs) in a gene determines the amino acid sequence of the protein. DNA sequencing is the process of determining the sequence of nucleotides in a piece of DNA.
Choice A is incorrect because enzymes are proteins that catalyze chemical reactions, not DNA sequences. Choice B is incorrect because blood types are determined by the presence or absence of certain antigens on the surface of red blood cells, not by DNA sequences. Choice C is incorrect because hormones are chemical messengers that regulate various body functions, not DNA sequences.
Correct Answer is C
Explanation
During ovulation, a mature egg is released from the female ovary, enabling it to be fertilized by male sperm cells.
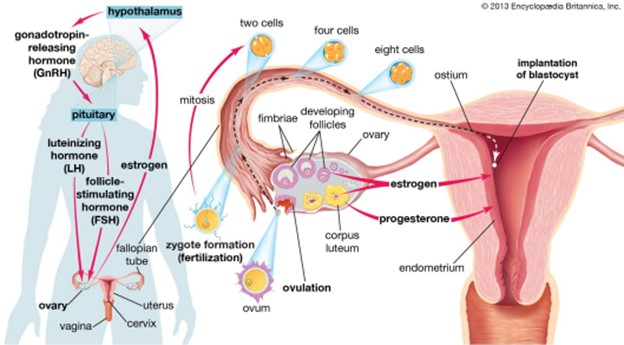
Choice A is incorrect because menstruation is the process of shedding the uterine lining, which occurs when an egg is not fertilized.
Choice B is incorrect because fertilization is the process of a sperm cell joining with an egg cell to form a zygote.
Choice D is incorrect because oogenesis is the process of forming female gametes (eggs) in the ovaries.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
