What is hydrogen bonding?
The attraction between one molecule's relatively positive areas and another's relatively negative areas.
The repulsion between the positive and negative charges of two molecules.
The attraction between two nonpolar molecules.
The attraction between two ionic molecules.
The Correct Answer is A
Hydrogen bonding is an interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons.
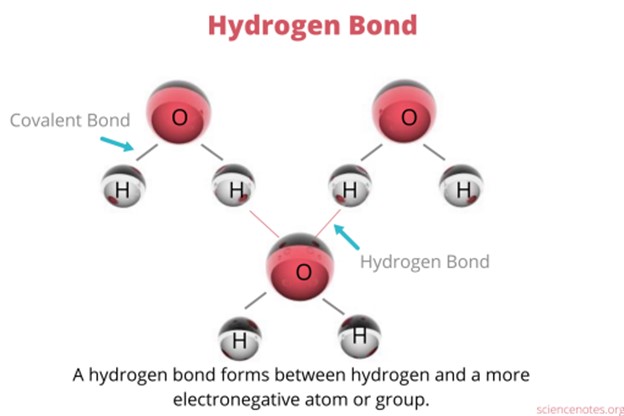
One atom of the pair (the donor), generally a fluorine, nitrogen, or oxygen atom, is covalently bonded to a hydrogen atom, whose electrons it shares unequally; its high electron affinity causes the hydrogen to take on a slight positive charge.
The other atom of the pair (the acceptor), also typically F, N, or O, has an unshared electron pair, which gives it a slight negative charge.
Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond.
Choice B) The repulsion between the positive and negative charges of two molecules is incorrect because hydrogen bonding involves attraction, not repulsion.
Choice C) The attraction between two nonpolar molecules is incorrect because hydrogen bonding involves polar molecules.
Choice D) The attraction between two ionic molecules is incorrect because hydrogen bonding involves polar molecules and not ionic molecules.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
Orthopnea refers to a condition in which a patient experiences difficulty breathing while lying down, but their breathing improves when they sit up or stand.
Choice B, Hypoxia, is not the correct answer because it refers to a condition in which there is a lack of oxygen supply to the body’s tissues.
Choice C, Tachypnea, is not the correct answer because it refers to rapid breathing.
Choice D, Bradypnea, is not the correct answer because it refers to abnormally slow breathing.
Correct Answer is B
Explanation
Using a placebo group and a double-blind technique for giving the medications is the best way to ensure that the study is valid and reliable.
A placebo group helps control for the placebo effect, which can influence the results of a study.
A double-blind technique means that neither the patients nor the researchers know which medication is being given, reducing bias.
Choice A is not the best answer because while a large sample size and standardized procedure can increase reliability, they do not address validity.
Choice C is not the best answer because a matched-pairs design and crossover technique are useful for reducing variability but do not address validity.
Choice D is not the best answer because a convenience sample may not be representative and a pretest-posttest design does not control for extraneous variables.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
