In a phase diagram, which of the following is the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously?
Triple point.
Critical temperature.
Critical point.
Absolute zero.
The Correct Answer is A
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
The threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential.
Most often, the threshold potential is a membrane potential value between –50 and –55 mV.
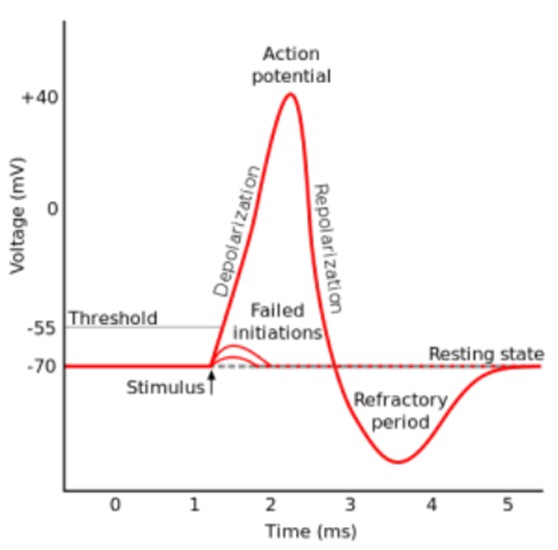
The membrane potential of a neuron is determined by the distribution of ions across the cell membrane.
At rest, the inside of a neuron is more negative than the outside due to the presence of negatively charged proteins and other molecules.
The movement of ions across the cell membrane can change the membrane potential.
For example, when sodium ions enter the cell, they make the inside of the cell more positive (less negative), causing depolarization.
Choice B is incorrect because -80 mV is below the typical threshold value for mammalian neurons.
Choice C is incorrect because +35 mV is above the typical threshold value for mammalian neurons.
Choice D is incorrect because 0 mV is above the typical threshold value for mammalian neurons.
Correct Answer is B
Explanation
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.
Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
