A nurse is reviewing the laboratory values of a client who has respiratory acidosis. Which of the following findings should the nurse expect?
Potassium 3.3 mEq/L.
HCO3- 30 mEq/L.
PacO2 50 mm Hg.
pH 7.45.
The Correct Answer is C
PacO2 50 mm Hg. Choice A rationale:
Potassium levels are not directly related to respiratory acidosis. Potassium levels may be affected in certain conditions, but they are not specific indicators of respiratory acidosis.
Choice B rationale:
HCO3- (bicarbonate) levels may be elevated in metabolic alkalosis, not respiratory acidosis. In respiratory acidosis, the primary abnormality is an increased PacO2, not HCO3-.
Choice C rationale:
The partial pressure of carbon dioxide (PacO2) is a key parameter in diagnosing respiratory acidosis. In this case, a PacO2 of 50 mm Hg suggests hypoventilation and an excess of carbon dioxide in the blood, contributing to acidosis.
Choice D rationale:
The pH level given (pH 7.45) is within the normal range, which contradicts the diagnosis of respiratory acidosis. In respiratory acidosis, the pH would be expected to be below the normal range of 7.35-7.45 due to increased carbon dioxide levels.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
The correct answer is B. 600 milliliters.
Choice A rationale:
A 24-hour urine output of 1000 milliliters is within the normal range for an adult, indicating adequate kidney function and hydration.
Choice B rationale:
A 24-hour urine output of 600 milliliters is below the normal range (typically 800-2000 milliliters), which may indicate oliguria (reduced urine output) and could be a sign of renal impairment or dehydration. This warrants notifying the healthcare provider.
Choice C rationale:
A 24-hour urine output of 1200 milliliters is also within the normal range, suggesting normal kidney function and hydration status.
Choice D rationale:
A 24-hour urine output of 750 milliliters is slightly below the normal range but may not be immediately concerning unless accompanied by other symptoms. However, it is still important to monitor and possibly notify the healthcare provider if it persists.
Correct Answer is B
Explanation
Cerebral bleeding. Choice A rationale:
Stress fractures are not directly related to hypernatremia. Hypernatremia is an electrolyte imbalance, and its main effects are related to cellular dehydration and neurological symptoms rather than bone fractures.
Choice B rationale:
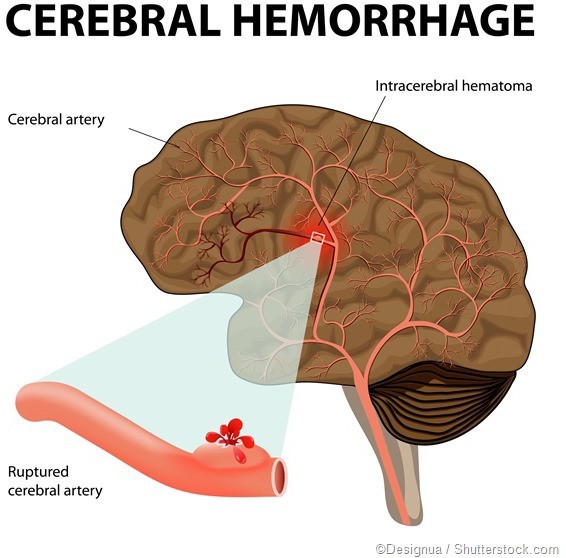
This is the correct answer because hypernatremia can lead to severe dehydration and cause neurological complications, including cerebral bleeding. The brain cells can shrink due to water loss, causing blood vessels to rupture, leading to bleeding in the brain.
Choice C rationale:
Atrial dysrhythmias are not directly associated with hypernatremia. Hypernatremia primarily affects the central nervous system and can lead to neurological symptoms rather than cardiac dysrhythmias.
Choice D rationale:
Pulmonary edema is not a likely consequence of hypernatremia. Pulmonary edema is associated with fluid volume excess, not fluid volume deficit, which is characteristic of hypernatremia.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
