A nurse is reviewing the laboratory data of a client who is receiving filgrastim. Which of the following laboratory values should the nurse monitor to evaluate the effectiveness of the treatment?
INR
BUN
WBC count
Potassium level
The Correct Answer is C
Choice A Reason:
INR (International Normalized Ratio) is incorrect. INR is a measurement used to monitor the effects of anticoagulants like warfarin. It assesses the blood's ability to clot. Filgrastim does not directly affect INR levels, so monitoring INR would not provide information about the effectiveness of filgrastim in stimulating white blood cell production.
Choice B Reason:
BUN (Blood Urea Nitrogen) is incorrect. BUN levels primarily indicate kidney function and hydration status. They are not directly influenced by filgrastim treatment. Monitoring BUN is essential for assessing kidney function but does not reflect the effectiveness of filgrastim therapy in increasing white blood cell counts.
Choice C Reason:
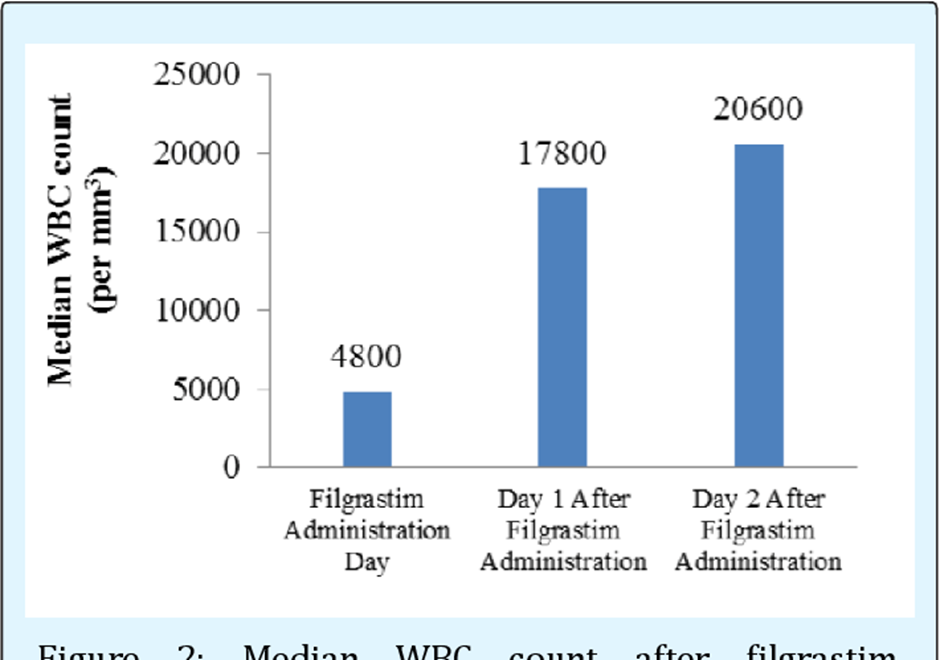
WBC count is correct. Filgrastim is a medication that stimulates the production of white blood cells (WBCs), particularly neutrophils. Therefore, the key laboratory value to monitor for assessing the effectiveness of filgrastim therapy is the WBC count. An increase in the WBC count, particularly in the neutrophil count, signifies the intended therapeutic effect of filgrastim in boosting the immune system's response by increasing the production of these infection-fighting cells.
Choice D Reason:
Potassium level is incorrect. Potassium levels are crucial for heart and muscle function. While certain medications might affect potassium levels, filgrastim's primary action is on stimulating white blood cell production and does not directly impact potassium levels. Monitoring potassium levels is essential for overall health but does not specifically indicate the effectiveness of filgrastim treatment.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
Choice A Reason:
Furosemide is not correct. Furosemide is a diuretic used to treat conditions like heart failure and edema by increasing urine output. It is not a contraindication for sildenafil. However, it's essential to monitor blood pressure when these medications are used together, as both can potentially lower blood pressure.
Choice B Reason:
Albuterol is not correct. Albuterol is a bronchodilator commonly used to treat asthma and other respiratory conditions. It doesn't have direct contraindications with sildenafil for erectile dysfunction. Although both medications can cause some cardiovascular effects, they are not typically considered contraindications for each other.
Choice C Reason:
Indomethacin is not correct. Indomethacin is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce inflammation and pain. While it can have effects on blood pressure and the cardiovascular system, it is not a direct contraindication for sildenafil specifically for erectile dysfunction.
Choice D Reason:
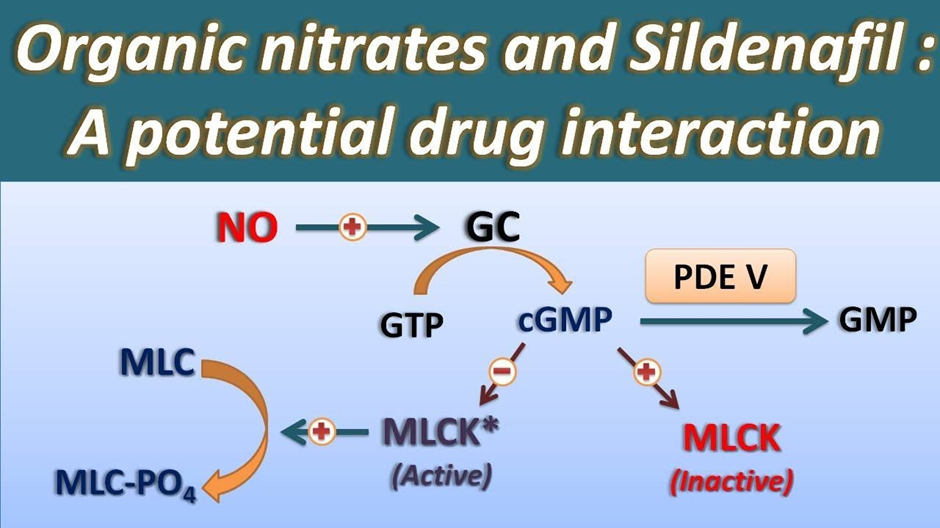
Nitroglycerin is correct. Nitroglycerin is a contraindication for sildenafil. Both medications can cause a significant drop in blood pressure. When taken together, they can potentiate each other's effects, leading to a severe decrease in blood pressure, which can be dangerous and potentially life-threatening. Therefore, individuals using nitroglycerin or any nitrate medications should not take sildenafil or other medications for erectile dysfunction due to the risk of hypotension (dangerously low blood pressure).
Correct Answer is D
Explanation
Choice A Reason:
Weight gain is incorrect. Weight gain is typically associated with fluid volume excess rather than deficit. In heart failure, fluid retention can lead to weight gain due to excess fluid accumulation in the body.
Choice B Reason:
Distended neck veins is incorrect. Distended neck veins are a sign of fluid volume excess, commonly seen in heart failure due to increased venous pressure and fluid retention.
Choice C Reason:
Shortness of breath is incorrect.: Shortness of breath is often associated with fluid accumulation in the lungs, known as pulmonary edema, which is a manifestation of fluid volume excess or fluid overload in heart failure.
Choice D Reason:
Elevated hematocrit level is correct. Furosemide is a diuretic that promotes diuresis (increased urine output), leading to fluid loss. When a client experiences fluid volume deficit or dehydration due to increased diuresis, there is a concentration of red blood cells in the blood, resulting in an elevated hematocrit level. This occurs because the blood becomes more concentrated when there's less fluid volume available.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
