A nurse instructs the patient that if ciprofloxacin (Cipro) is taken with antacids, absorption is
Not affected
delayed
increased
decreased
The Correct Answer is D
A. Not affected:
This choice suggests that taking ciprofloxacin with antacids does not alter its absorption. However, this is not correct. When ciprofloxacin is taken with antacids containing certain ions (such as aluminum, magnesium, or calcium), the absorption of ciprofloxacin can indeed be affected due to the formation of insoluble complexes, leading to decreased absorption.
B. Delayed:
This choice implies that taking ciprofloxacin with antacids delays its absorption. While it's true that the interaction between ciprofloxacin and certain antacids can alter absorption, the main effect is not typically a delay in absorption but rather a decrease due to the formation of insoluble complexes. Therefore, while "delayed" may somewhat describe the effect, it doesn't fully capture the nature of the interaction.
C. Increased:
This choice suggests that taking ciprofloxacin with antacids increases its absorption. However, this is not accurate. Antacids containing aluminum, magnesium, or calcium can interfere with the absorption of ciprofloxacin by forming insoluble complexes with the drug, leading to decreased absorption rather than an increase.
D. Decreased:
This choice correctly identifies the effect of taking ciprofloxacin with antacids. When ciprofloxacin is taken concurrently with antacids containing aluminum, magnesium, or calcium, the absorption of ciprofloxacin is decreased. The ions in the antacids bind with ciprofloxacin in the gastrointestinal tract, forming insoluble complexes that are poorly absorbed, thereby reducing the effectiveness of the antibiotic.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
A. Headaches:
Headaches are a common symptom that can occur for various reasons, including stress, tension, dehydration, or as a side effect of medications. While headaches can sometimes occur as a side effect of certain drugs, they are not specific indicators of a drug allergy. Allergic reactions to medications typically involve other symptoms such as rash, hives, itching, swelling, or respiratory symptoms.
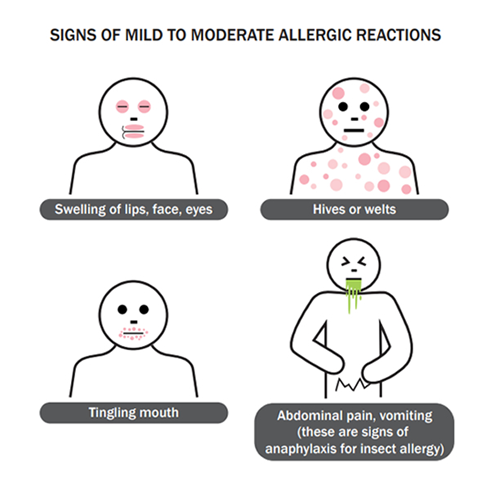
B. Hives or shortness of breath:
Hives (urticaria) are raised, red, itchy welts on the skin that can occur as an allergic reaction to medications. They are a common manifestation of drug allergies. Shortness of breath (dyspnea) can occur as part of a severe allergic reaction known as anaphylaxis. Anaphylaxis is a life-threatening allergic reaction characterized by a rapid onset of symptoms, including difficulty breathing, swelling of the throat or tongue, rapid heart rate, and low blood pressure. Both hives and shortness of breath are significant signs of a potential drug allergy and require immediate attention.
C. Diarrhea:
Diarrhea can occur as a side effect of medications, including antibiotics. However, it is not typically a specific indicator of a drug allergy. Diarrhea is more commonly associated with gastrointestinal disturbances or as a reaction to changes in gut flora due to antibiotic use.
D. Nausea:
Nausea is a common side effect of many medications, including antibiotics. While it can be bothersome, nausea alone is not a specific indicator of a drug allergy. Allergic reactions to medications typically involve other symptoms such as rash, hives, itching, swelling, or respiratory symptoms.
Correct Answer is B
Explanation
A. Decision to administer either a bactericidal or bacteriostatic drug:
Culture and sensitivity tests provide information about the susceptibility of the microorganism to specific antimicrobial agents. Based on this information, healthcare providers can choose between bactericidal (agents that kill bacteria) or bacteriostatic (agents that inhibit bacterial growth) drugs. For example, if the culture indicates that the microorganism is susceptible to a bactericidal drug, such as penicillin, the healthcare provider may choose to administer that type of drug.
B. Microbial susceptibility to an anti-infective:
This option accurately describes one of the primary purposes of culture and sensitivity tests. These tests determine whether the microorganism causing the infection is susceptible or resistant to specific antimicrobial agents. This information guides the selection of the most appropriate anti-infective therapy to effectively treat the infection.
C. Duration of the antibacterial drug therapy:
While culture and sensitivity tests provide valuable information about microbial susceptibility to antimicrobial agents, they do not specifically determine the duration of antibacterial drug therapy. The duration of therapy is often determined based on factors such as the type and severity of the infection, the patient's response to treatment, and clinical guidelines, rather than solely on the results of culture and sensitivity tests.
D. Decision to administer empiric therapy:
Empiric therapy involves the initiation of antimicrobial treatment based on clinical judgment and knowledge of likely pathogens before culture and sensitivity results are available. Culture and sensitivity tests help confirm the causative microorganism and guide subsequent treatment decisions, including adjustments to therapy based on the results. Therefore, while culture and sensitivity tests inform decisions regarding antimicrobial therapy, they do not directly determine whether empiric therapy should be initiated.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
