Which of the following properties of water explains its solvent abilities for certain substances?
Kinetic energy of liquid water molecules
High specific heat
High surface tension
Polarity of water molecules.
The Correct Answer is D
The polarity of water molecules explains its solvent abilities for certain substances.
Water is a polar molecule because it has a partial positive charge on one end and a partial negative charge on the other end due to the unequal sharing of electrons between the oxygen and hydrogen atoms.
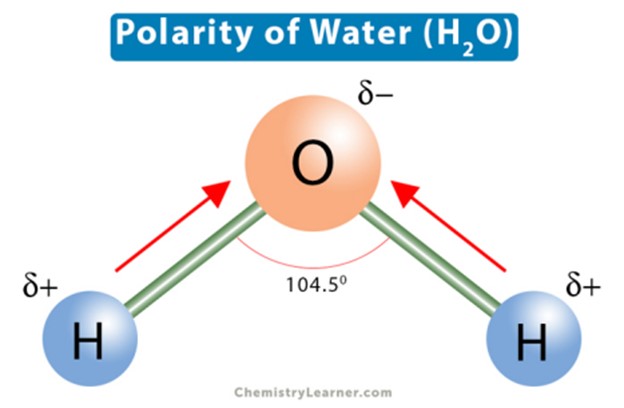
This polarity allows water to dissolve other polar substances and ionic compounds.
Choice A. Kinetic energy of liquid water molecules is not the correct answer because kinetic energy refers to the energy of motion and does not directly explain water’s solvent abilities.
Choice B. High specific heat is not the correct answer because specific heat refers to the amount of heat required to raise the temperature of a substance and does not directly explain water’s solvent abilities.
Choice C. High surface tension is not the correct answer because surface tension refers to the cohesive forces between liquid molecules and does not directly explain water’s solvent abilities.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
Correct Answer is D
Explanation
Testosterone is classified as an androgen hormone.
Androgens are a type of sex hormone that primarily regulates the development and maintenance of male characteristics, such as body hair growth, muscle mass, and deepening of the voice.
Testosterone is produced primarily in the testes in males and in smaller amounts in the ovaries and adrenal glands in females.
Option A, estrogen, is a female hormone that regulates the development of female sexual characteristics, such as breast growth and menstruation.
While estrogen and testosterone are both steroid hormones and can be converted to one another in the body, testosterone is not categorized as estrogen.
Option B, progestin, is a synthetic form of the hormone progesterone.
Progesterone is a female hormone that plays a role in the menstrual cycle and pregnancy.
Testosterone and progestin are not related, and testosterone is not categorized as progestin.
Option C, aldosterone, is a mineralocorticoid hormone that regulates salt and water balance in the body.
It is produced in the adrenal gland and plays a role in regulating blood pressure.
Testosterone and aldosterone are not related, and testosterone is not categorized as aldosterone.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
