An electrolyte is a mineral or salt dissolved in the body. (Write True or False)
True
False
The Correct Answer is A
Choice A reason: This is correct because an electrolyte is a substance that dissociates into ions when dissolved in water, and can conduct electricity. Some examples of electrolytes in the body are sodium, potassium, calcium, chloride, and bicarbonate.
Choice B reason: This is incorrect because an electrolyte is not a solid or a gas, but a dissolved substance. Electrolytes are essential for maintaining fluid balance, nerve impulses, muscle contractions, and acid-base balance in the body.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is C
Explanation
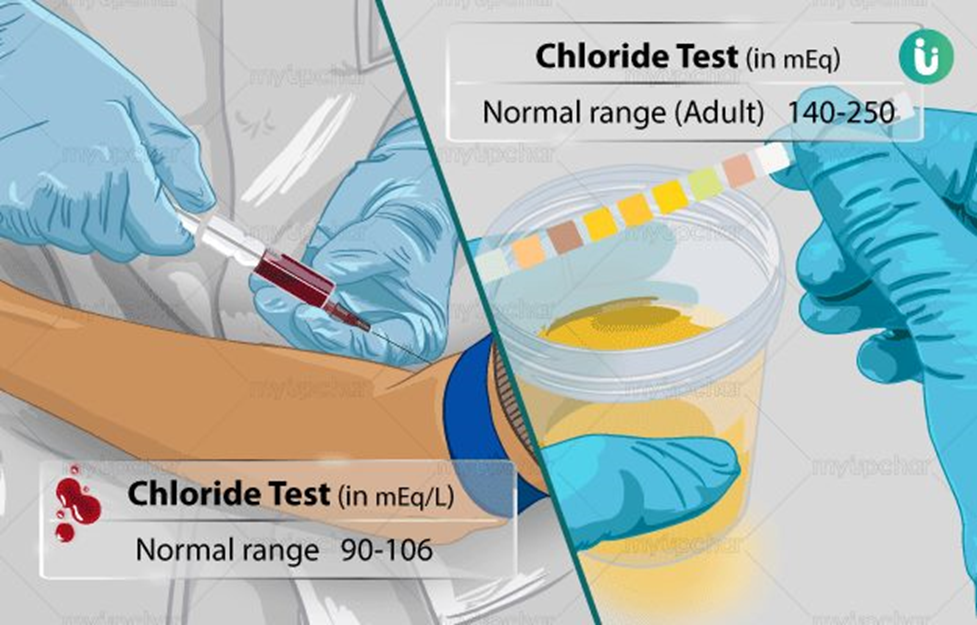
Choice A reason: This is incorrect because 95-110 mg/dL is the normal range of serum phosphorus level in adults, not chloride. Phosphorus is an electrolyte that is involved in energy metabolism, acid-base balance, and bone formation.
Choice B reason: This is incorrect because 10-120 mEq/L is not a realistic range for any electrolyte level in the blood. The units of mEq/L indicate the concentration of ions, not the mass of the substance. The normal range of serum chloride level in adults is expressed in mEq/L, not mg/dL.
Choice C reason: This is correct because 96-106 mEq/L is the normal range of serum chloride level in adults. Chloride is an electrolyte that is important for fluid balance, acid-base balance, and nerve transmission.
Choice D reason: This is incorrect because 1.8-2.6 mEq/L is the normal range of serum magnesium level in adults, not chloride. Magnesium is an electrolyte that is important for muscle and nerve function, as well as enzyme activity.
Correct Answer is D
Explanation
Choice A reason: This is incorrect because the adrenal gland is not involved in the thirst mechanism. The adrenal gland is responsible for producing hormones such as cortisol, aldosterone, and adrenaline, which regulate stress response, blood pressure, and metabolism.
Choice B reason: This is incorrect because the cerebral cortex is not involved in the thirst mechanism. The cerebral cortex is the outer layer of the brain that is responsible for higher cognitive functions such as memory, language, and reasoning.
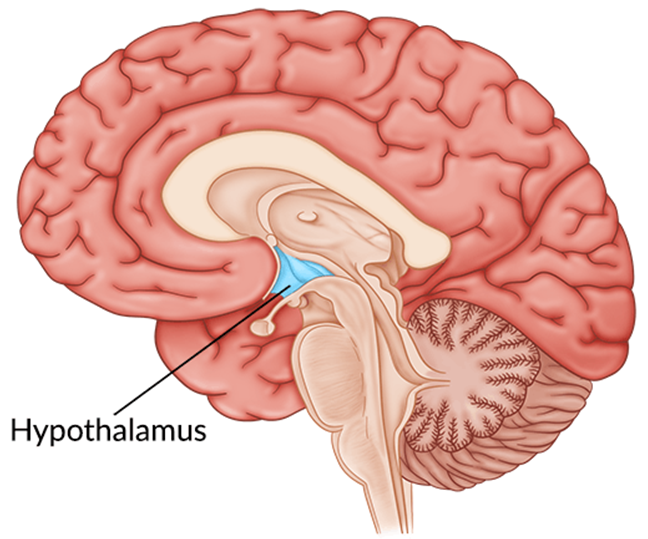
Choice C reason: This is incorrect because the pituitary gland is not directly involved in the thirst mechanism. The pituitary gland is a small gland at the base of the brain that produces hormones that control growth, reproduction, and metabolism. However, the pituitary gland does secrete antidiuretic hormone (ADH), which is regulated by the hypothalamus and affects water balance in the body.
Choice D reason: This is correct because the hypothalamus is the location of the thirst mechanism. The hypothalamus is a part of the brain that regulates many bodily functions such as temperature, appetite, sleep, and emotions. The hypothalamus also monitors the blood osmolarity and triggers the sensation of thirst when the blood is too concentrated.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
