Which of the following processes is required for normal blood clotting?
Activation of plasmin
Activation of heparin
Adequate levels of potassium
Adequate levels of calcium
The Correct Answer is D
a. Activation of plasmin: Plasmin is involved in breaking down blood clots, not in their formation. It is part of the fibrinolytic system.
b. Activation of heparin: Heparin is an anticoagulant that prevents clotting; it is not required for blood clotting.
c. Adequate levels of potassium: Potassium is important for cellular function but does not play a direct role in blood clotting.
d. Adequate levels of calcium: Calcium ions are essential for various steps in the blood clotting cascade, including the activation of certain clotting factors.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is B
Explanation
a. Cell body: The cell body is the main part of the neuron containing the nucleus and other organelles. It does not directly transmit impulses.
b. Axon: The axon is a long fiber that carries electrical impulses away from the cell body towards other neurons or muscles.
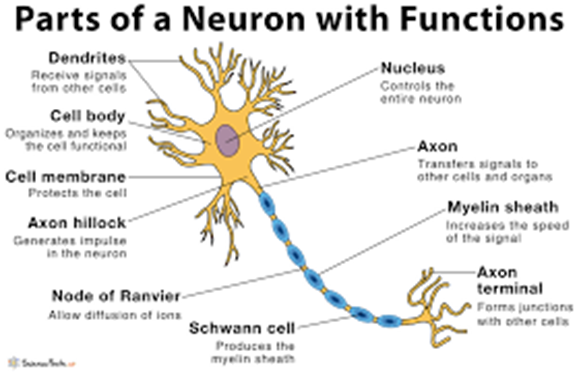
c. Synapse: A synapse is a junction between two neurons where nerve impulses are transmitted. The axon of one neuron transmits an electrical signal to the dendrite or cell body of another neuron across a small gap. Neurotransmitters are released from the presynaptic neuron and bind to receptors on the postsynaptic neuron, either exciting or inhibiting it.
d. Dendrite: Dendrites are short, branching fibers that receive signals from other neurons and transmit them towards the cell body.
Correct Answer is C
Explanation
a. Ovary: The ovary releases the ovum during ovulation, but fertilization does not occur here.
b. Uterus: The fertilized ovum implants and develops in the uterus, but fertilization occurs earlier.
c. Fallopian tube: Fertilization typically occurs in the ampulla of the fallopian tube, where the sperm meets the ovum.
d. Cervix: The cervix is the lower part of the uterus and is not involved in the fertilization process.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
