Which of the following is a chemical property of a substance?
Density
Melting point
Boiling point
Reactivity with acid
The Correct Answer is D
Chemical properties are characteristics of a substance that describe its ability to undergo a chemical change or reaction with another substance. Reactivity with acid is a chemical property because it describes how a substance will react with an acid to produce a new substance. Density, melting point, and boiling point are physical properties that describe how a substance behaves under certain conditions but do not involve a chemical change or reaction.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
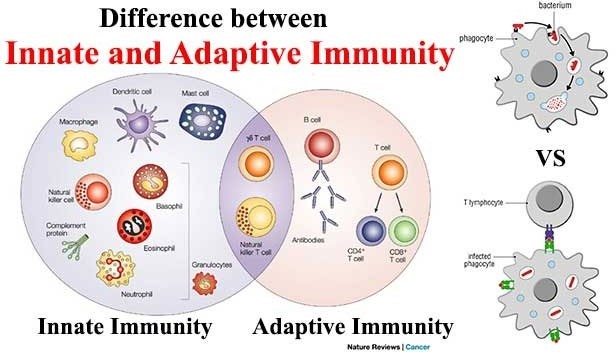
Innate immunity and adaptive immunity are two arms of the immune system that work together to protect the body from pathogens. Innate immunity is the first line of defense and is present at birth. It includes physical and chemical barriers such as the skin, mucous membranes, and antimicrobial peptides, as well as cells such as macrophages and natural killer cells that can quickly recognize and attack pathogens. Innate immunity is nonspecific, meaning it responds to a wide variety of pathogens in a similar way.
Adaptive immunity, on the other hand, is acquired after exposure to pathogens. It involves the production of antibodies and activation of T cells, which are specific to particular pathogens. Adaptive immunity takes longer to develop than innate immunity, but it provides a more specific and targeted response to pathogens. Once the adaptive immune system has been activated against a particular pathogen, it can provide long-term protection against future infections with that pathogen.
Option b) is incorrect because innate immunity is nonspecific while adaptive immunity is specific.
Option c) is incorrect because antibodies are a part of adaptive immunity while T cells can be a part of both innate and adaptive immunity.
Option d) is incorrect because adaptive immunity can provide long-term protection, while innate immunity provides immediate but short-lived protection.
Correct Answer is B
Explanation
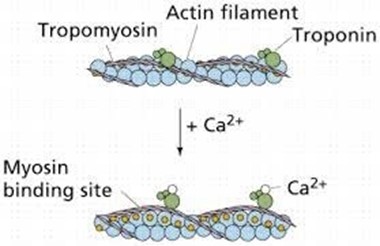
Muscle contraction is a complex process that involves the interaction between actin and myosin filaments in the muscle fibers. The sliding of these filaments is initiated by the release of calcium ions from the sarcoplasmic reticulum, a specialized organelle in muscle cells. The calcium ions bind to the protein troponin, which causes a conformational change in the troponin-tropomyosin complex, exposing the myosin-binding sites on actin. This allows the myosin heads to bind to actin, forming cross-bridges that pull the actin filaments toward the center of the sarcomere, resulting in muscle contraction.
Option a) is incorrect because calcium does not bind to tropomyosin directly, but rather binds to the protein troponin, causing a conformational change in the troponin-tropomyosin complex.
Option c) is incorrect because calcium does not activate motor neurons, but rather is released from the sarcoplasmic reticulum in response to an action potential that travels down the motor neuron to the neuromuscular junction.
Option d) is incorrect because calcium is required for muscle contraction, not relaxation. The relaxation of muscles after contraction is due to the active transport of calcium ions back into the sarcoplasmic reticulum, which allows the troponin-tropomyosin complex to return to its resting conformation, blocking the myosin-binding sites on actin and ending the cross-bridge cycle.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
