What are the five regions of the vertebral column, starting from the top and moving downwards?
Cervical, thoracic, lumbar, sacral, coccygeal
Thoracic, cervical, lumbar, sacral, coccygeal
Lumbar, thoracic, cervical, coccygeal, sacral
Sacral, lumbar, cervical, thoracic, coccygeal
The Correct Answer is A
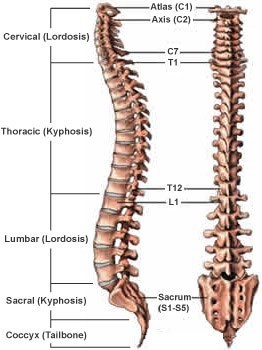
The vertebral column, also known as the spine or spinal column, is a series of bones called vertebrae that extend from the skull to the pelvis. It provides support for the body and protects the spinal cord. The five regions of the vertebral column, starting from the top and moving downwards, are:
- Cervical: This region is made up of seven vertebrae and is located in the neck. The first two cervical vertebrae, the atlas, and the axis are specialized to allow for head movement.
- Thoracic: This region is made up of twelve vertebrae and is located in the upper and middle back. The thoracic vertebrae are larger than the cervical vertebrae and articulate with the ribs.
- Lumbar: This region is made up of five vertebrae and is located in the lower back. The lumbar vertebrae are the largest and strongest of the vertebrae.
- Sacral: This region is made up of five fused vertebrae and is located in the pelvis. The sacrum forms the posterior wall of the pelvis and articulates with the hip bones.
- Coccygeal: This region is made up of four fused vertebrae and is located at the base of the vertebral column. The coccyx, or tailbone, provides attachment points for muscles and ligaments.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
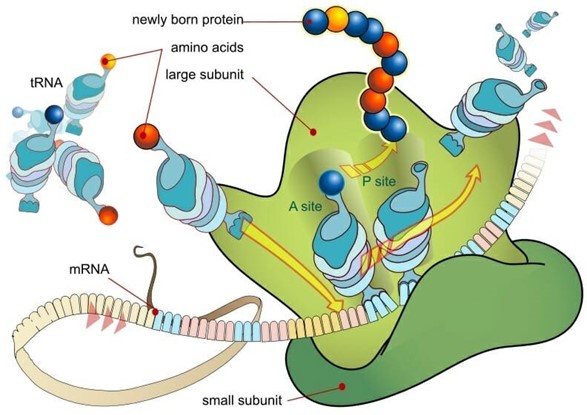
Ribosomes are small, spherical structures found in all living cells, including bacteria, archaea, and eukaryotes. Their primary function is to synthesize proteins using the genetic information stored in the cell's DNA. Ribosomes are composed of two subunits, one large and one small, that come together during protein synthesis.
Ribosomes read the genetic information stored in mRNA (messenger RNA) and use this information to assemble amino acids in the correct order to form a protein. The ribosome moves along the mRNA, adding one amino acid at a time to the growing protein chain until it reaches the end of the mRNA and the protein is complete.
Proteins are essential for a wide variety of cellular functions, including catalyzing chemical reactions, providing structural support, and transporting molecules across cell membranes. Therefore, ribosomes play a critical role in the overall function and survival of a cell.
Correct Answer is A
Explanation
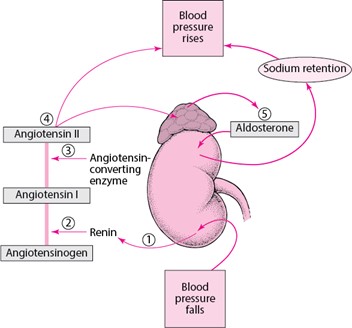
Renin is an enzyme that is produced by the kidneys and it acts to elevate blood pressure. When blood pressure falls, the kidneys secrete renin into the bloodstream
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
