What is the difference between isotonic and isometric muscle contractions?
Isotonic contractions produce no movement while isometric contractions produce movement.
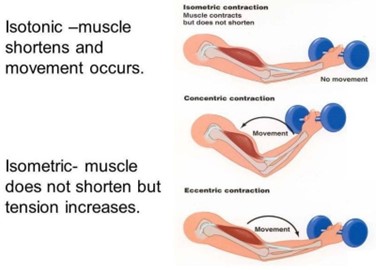
Isotonic contractions produce movement while isometric contractions produce no movement.
Isotonic contractions generate tension in the muscle while isometric contractions involve the shortening of the muscle fibers.
Isotonic contractions involve the contraction of individual muscle fibers while isometric contractions involve the entire muscle.
The Correct Answer is B
Isotonic and isometric contractions are two types of muscle contractions that differ in the amount of force produced and the movement of the muscle. In isotonic contractions, the muscle changes length and produces movement, such as lifting a weight. The force generated by the muscle remains constant throughout the movement. Isotonic contractions can be further classified as concentric contractions, in which the muscle shortens as it contracts, and eccentric contractions, in which the muscle lengthens as it contracts.
In contrast, isometric contractions occur when the muscle generates force without changing its length or producing movement. For example, holding a weight in a fixed position without moving it requires an isometric contraction. In an isometric contraction, the force generated by the muscle increases up to a maximum and then remains constant. Isometric contractions can be used to build strength and endurance in the muscle, but they do not produce movement.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is A
Explanation
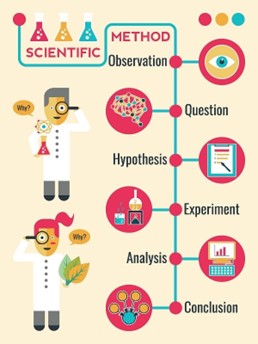
The scientific method is a systematic approach used to answer questions or test hypotheses about the natural world. The steps involved in the scientific method are:
- Observation: This is the first step in the scientific method. It involves observing a phenomenon or a problem and gathering information about it.
- Hypothesis: After making an observation, a scientist forms a hypothesis, which is a tentative explanation for the phenomenon or problem.
- Prediction: Based on the hypothesis, the scientist makes a prediction about what will happen in an experiment or what they will observe.
- Experimentation: The scientist designs and conducts an experiment to test the hypothesis and prediction.
- Analysis: The data collected from the experiment are analyzed to determine if they support or refute the hypothesis.
- Conclusion: Based on the analysis of the data, the scientist draws a conclusion about whether the hypothesis is supported or refuted.
Option b) is incorrect because it starts with hypothesis before observation.
Option c) is incorrect because prediction comes before experimentation.
Option d) is incorrect because hypothesis comes after observation and data collection.
Correct Answer is A
Explanation
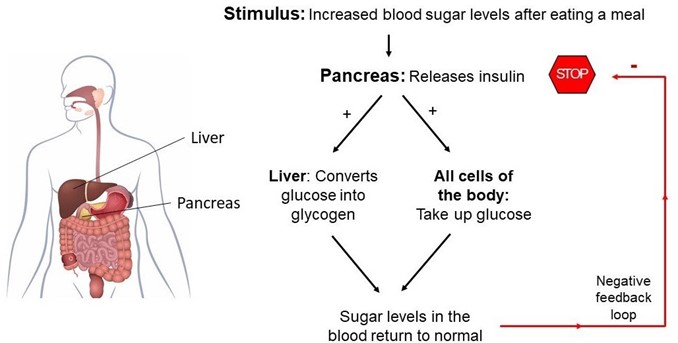
Insulin is a hormone produced by the pancreas that plays a crucial role in regulating the levels of glucose (sugar) in the blood. After a person eats a meal, the levels of glucose in the blood rise, which stimulates the pancreas to release insulin into the bloodstream. Insulin acts on various cells in the body, particularly those in the liver, muscles, and adipose tissue, to promote the uptake, use, and storage of glucose.
Insulin helps to lower the levels of glucose in the blood by increasing the uptake of glucose by cells, stimulating the liver and muscle cells to store glucose in the form of glycogen, and inhibiting the production and release of glucose by the liver. This process is known as glucose homeostasis, and it helps to keep the levels of glucose in the blood within a normal range.
Deficiencies or abnormalities in insulin production or function can lead to a range of metabolic disorders, including type 1 and type 2 diabetes. In type 1 diabetes, the body does not produce enough insulin, while in type 2 diabetes, the body becomes resistant to the effects of insulin, leading to elevated levels of glucose in the blood.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
