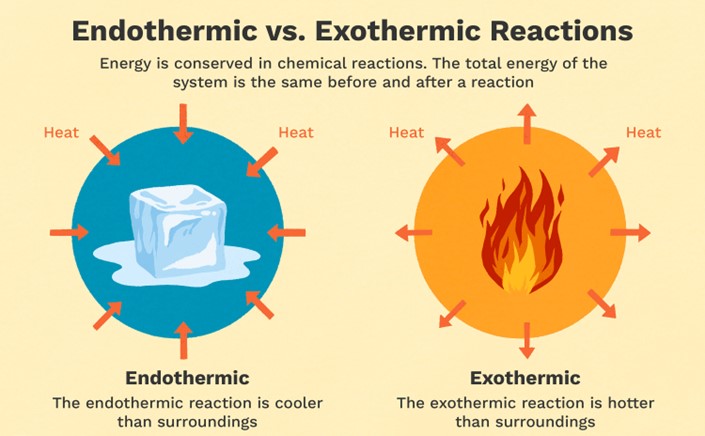
Which of the following is an example of an exothermic reaction?
Melting ice
Burning wood
Cooking an egg
Dissolving sugar in water
The Correct Answer is B
Exothermic reactions are reactions that release energy in the form of heat, light, or sound. Burning wood is an example of an exothermic reaction because it releases heat and light. As the wood reacts with oxygen in the air, it undergoes a combustion reaction that releases energy in the form of heat and light. Melting ice is an endothermic reaction because it requires energy input to melt the solid ice into liquid water. Cooking an egg is a chemical reaction that involves denaturing the proteins in the egg, but it is not necessarily exothermic or endothermic. Dissolving sugar in water is also not an example of an exothermic reaction because it does not release energy in the form of heat, light, or sound.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
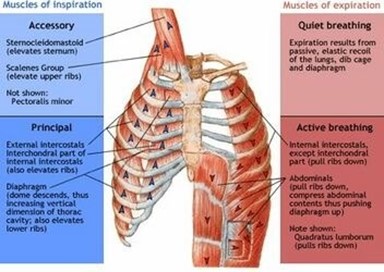
Correct Answer is A
Explanation
The diaphragm is a dome-shaped muscle that plays a key role in breathing. It separates the thoracic cavity, which contains the heart and lungs, from the abdominal cavity. When the diaphragm contracts, it moves downward and increases the volume of the thoracic cavity, allowing air to flow into the lungs. When it relaxes, it moves upward and decreases the volume of the thoracic cavity, forcing air out of the lungs.
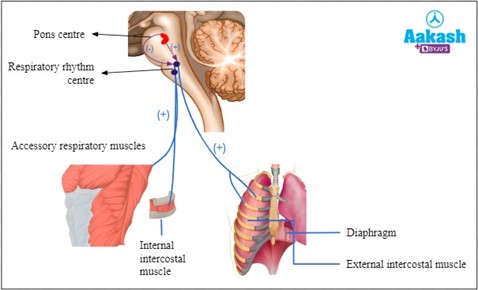
Correct Answer is C
Explanation
Diaphragm is responsible for regulating breathing rate and depth. It is a dome-shaped muscle located at the bottom of the chest cavity that contracts and relaxes to help move air in and out of the lungs.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
