Mrs. Kalen is a 70 year old female who has arrived into the ER due to persistent vomiting for two days now. She appears to be lethargic and weak and has myalgia. She is noted to have dry mucus membranes and her capillary refill takes >4 seconds. She is diagnosed as having gastroenteritis and dehydration. Measurement of arterial blood gas shows pH 7.5, PaO2 85 mm Hg, PaCO2 40 mm Hg, and HCO3 34 mmol/L. What would you interpret the acid-base disorder to be?
Uncompensated Respiratory Acidosis
Fully Compensated Metabolic Acidosis
Uncompensated Metabolic Alkalosis
Partially Compensated Respiratory Alkalosis
The Correct Answer is C
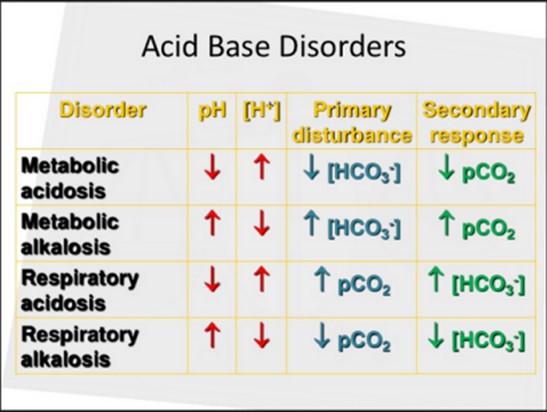
The pH value of 7.5 indicates alkalosis, as it is above the normal range of 7.35-7.45. The elevated bicarbonate (HCO3-) level of 34 mmol/L suggests metabolic alkalosis, as it is higher than the normal range of 22-28 mmol/L. The PaCO2 level of 40 mm Hg falls within the normal range of 35-45 mm Hg.
In this case, the primary disturbance is metabolic alkalosis, which is likely caused by vomiting leading to excessive loss of gastric acid (hydrogen ions) and chloride ions from the stomach. This loss of acid and chloride results in an imbalance of electrolytes and an increase in bicarbonate levels, leading to metabolic alkalosis.
The arterial blood gas results do not indicate any compensation. Compensation occurs when the body attempts to restore the pH balance by adjusting the respiratory or metabolic systems. In this case, there is no compensation observed because the PaCO2 level is within the normal range and not significantly altered.
Nursing Test Bank
Naxlex Comprehensive Predictor Exams
Related Questions
Correct Answer is D
Explanation
The red tag is used to identify patients with critical injuries who require immediate medical attention. These individuals have life-threatening conditions that, with prompt medical intervention, have a higher chance of survival.
The triage color code system typically follows the following priority order:
1. Red tag: Immediate or emergent care needed for life-threatening injuries or conditions.
2. Yellow tag: Urgent care needed for significant injuries or conditions that are not immediately life-threatening.
3. Green tag: Non-urgent care needed for minor injuries or illnesses that can wait for medical treatment.
4. Black tag: Comfort care or deceased, as the injuries or conditions are incompatible with life or resources are not available for treatment.
Correct Answer is A
Explanation
Effective teaching for a patient with peptic ulcer disease focuses on promoting understanding and adherence to the treatment plan, as well as addressing lifestyle modifications that can help manage the condition. Stress reduction is an important aspect of ulcer management, as stress
can exacerbate symptoms and delay healing. If the patient acknowledges learning relaxation strategies that decrease their stress, it indicates that they have grasped the concept and are likely to implement it in their daily life.
It is crucial for the patient to understand that any potential side effects should be reported to the healthcare provider, who will then determine the appropriate course of action. Abruptly stopping medications without medical guidance can have adverse consequences. While pain relief is an important goal, the treatment for peptic ulcer disease typically involves addressing the underlying cause, such as Helicobacter pylori infection or reducing stomach acid production. The patient should follow the treatment regimen as prescribed by the healthcare provider, even if they experience pain relief, to ensure proper healing and prevent ulcer recurrence.
Different antacids have different formulations and ingredients, and they may vary in terms of effectiveness and duration of action. It is important for the patient to consult with their healthcare provider or pharmacist to determine which antacid is most suitable for their specific needs.
Whether you are a student looking to ace your exams or a practicing nurse seeking to enhance your expertise , our nursing education contents will empower you with the confidence and competence to make a difference in the lives of patients and become a respected leader in the healthcare field.
Visit Naxlex, invest in your future and unlock endless possibilities with our unparalleled nursing education contents today
Report Wrong Answer on the Current Question
Do you disagree with the answer? If yes, what is your expected answer? Explain.
Kindly be descriptive with the issue you are facing.
