Which of the following units is used to indicate length?
kg
L
s
m
Correct Answer : D
The unit used to indicate length is the meter (m). It is the base unit of length in the International System of Units (SI).
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is C
Explanation
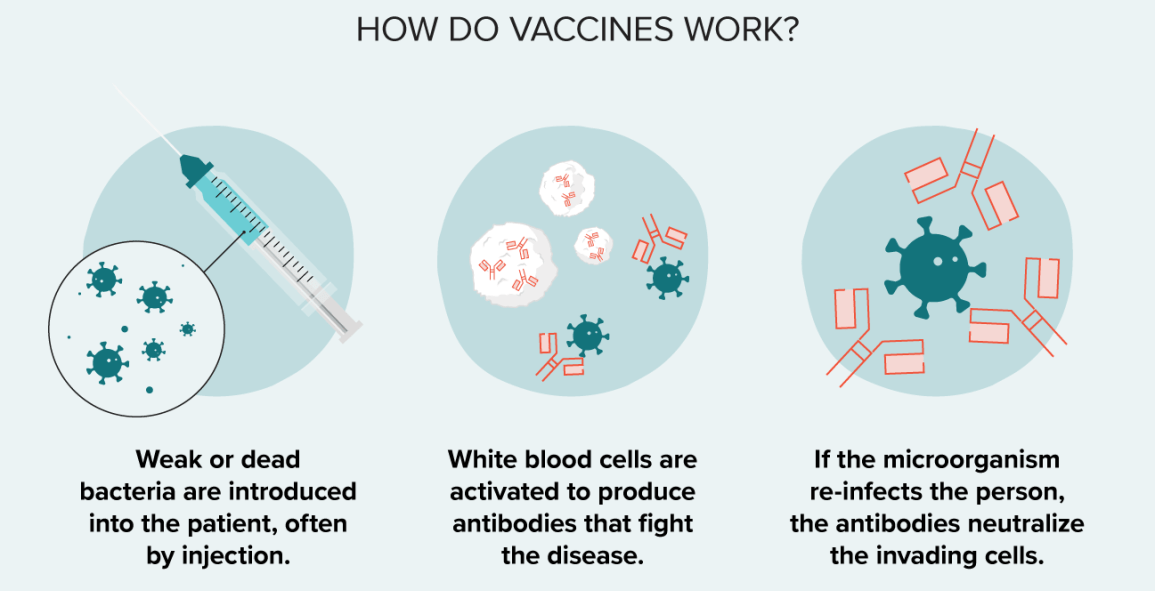
Vaccines are a type of preventative medicine that work by exposing the individual to a weakened or inactivated form of a pathogen (such as a virus or bacteria) or to a piece of the pathogen (such as a protein or sugar) that triggers an immune response in the body. This exposure allows the body to develop immunity to the pathogen without getting sick from the full-blown disease. Once the immune system has been primed, it can recognize and quickly respond to the pathogen if it is encountered again in the future, providing protection against the disease.
It is a common misconception that vaccines can cause the disease they are designed to protect against. This is not true. While some vaccines may cause mild symptoms such as a low-grade fever or soreness at the injection site, they do not cause the full-blown disease.
Vaccines provide active immunity, meaning that the body produces its own antibodies against the pathogen, rather than receiving pre-made antibodies as in passive immunity. Additionally, vaccines can be effective against both bacterial and viral infections, depending on the specific vaccine.
Correct Answer is C
Explanation
Facial acne is commonly caused by the blockage and inflammation of the sebaceous glands. These glands are responsible for secreting sebum, an oily substance that helps to lubricate the skin and hair. When these glands become overactive, or their ducts are blocked by excess sebum and dead skin cells, it can lead to the formation of pimples, blackheads, or whiteheads, which are typical signs of acne.
- Sebaceous glands are found near hair follicles and are primarily responsible for acne when they become clogged.
The other options are not related to acne:
- A. Lacrimal glands: These produce tears and are associated with the eye, not the skin.
- B. Sudoriferous glands: These are sweat glands, which are involved in perspiration, not typically linked to acne.
- D. Ceruminous glands: These are found in the ear canal and produce earwax, not involved in facial skin health.
Correct Answer is A
Explanation
Electrons are subatomic particles that possess a very small mass compared to protons and neutrons. The mass of an electron is approximately 11836of the mass of a proton or neutron, making it negligible when calculating the atomic mass of an atom.
In atomic mass calculations, protons and neutrons are considered because they make up the bulk of the atom's mass:
- Proton: Positively charged, and each proton has a mass of about 1 atomic mass unit (amu).
- Neutron: Neutral charge, with a mass also close to 1 amu.
- Electron: Negligible mass, contributing very little to the atomic mass, which is why the atomic mass number is typically determined by the number of protons and neutrons.
Quarks are the fundamental constituents of protons and neutrons but are not typically referred to in terms of atomic mass.
Correct Answer is A
Explanation
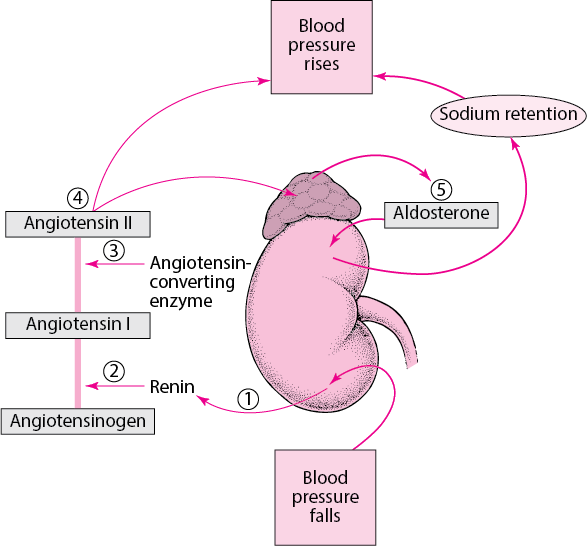
Renin is an enzyme that is produced by the kidneys and it acts to elevate blood pressure. When blood pressure falls, the kidneys secrete renin into the bloodstream ³.
 |
Correct Answer is A
Explanation
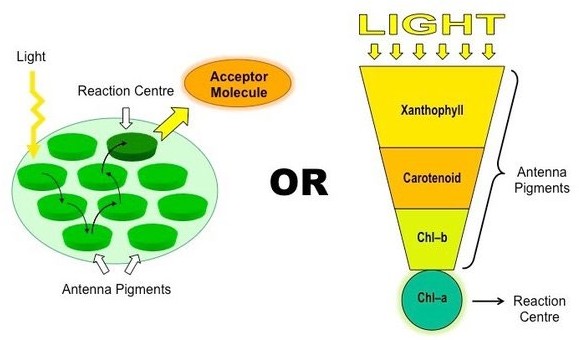
Chlorophyll a is the primary pigment responsible for photosynthesis in plants. It is a green pigment that is essential for capturing light energy from the sun and converting it into chemical energy that can be used by the plant. Chlorophyll a absorbs light most efficiently in the blue and red parts of the spectrum, and reflects green light, giving plants their characteristic green color
Chlorophyll b is another type of chlorophyll that is also involved in photosynthesis, but it is not as abundant as chlorophyll a. Chlorophyll b absorbs light most efficiently in the blue and orange parts of the spectrum and reflects yellow-green light.
Carotenoids are pigments that are present in many plants and are involved in photosynthesis as well as protecting the plant from damage caused by excess light. Carotenoids are responsible for the orange, yellow, and red colors of many fruits and vegetables.
Anthocyanins are pigments that give plants their red, purple, and blue colors. While they are not directly involved in photosynthesis, they play a role in atracting pollinators and protecting the plant from damage caused by UV radiation.
Correct Answer is D
Explanation
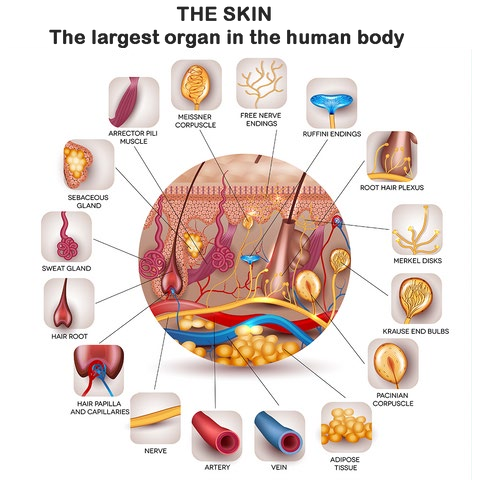
The largest organ in the human body by surface area is the skin. It covers the entire external surface of the body and has an average surface area of about 20 square feet in adults.
 |
Correct Answer is A
Explanation
A physical change is a change that affects the physical properties of a substance, but does not change its chemical identity. Physical changes include changes in state, such as melting or boiling, changes in shape or size, and changes in phase, such as the dissolution of a solid in a liquid. In a physical change, the atoms and molecules of the substance are rearranged, but no new substances are formed.
A chemical change, on the other hand, is a change that results in the formation of new substances with different chemical properties. Chemical changes involve the breaking of chemical bonds between atoms and the formation of new bonds to create new compounds. Chemical changes are usually accompanied by a change in color, the formation of a gas or a solid, or the release or absorption of energy.
Overall, the main difference between a physical change and a chemical change is that a physical change only affects the physical properties of a substance while a chemical change results in the formation of new substances with different chemical properties.
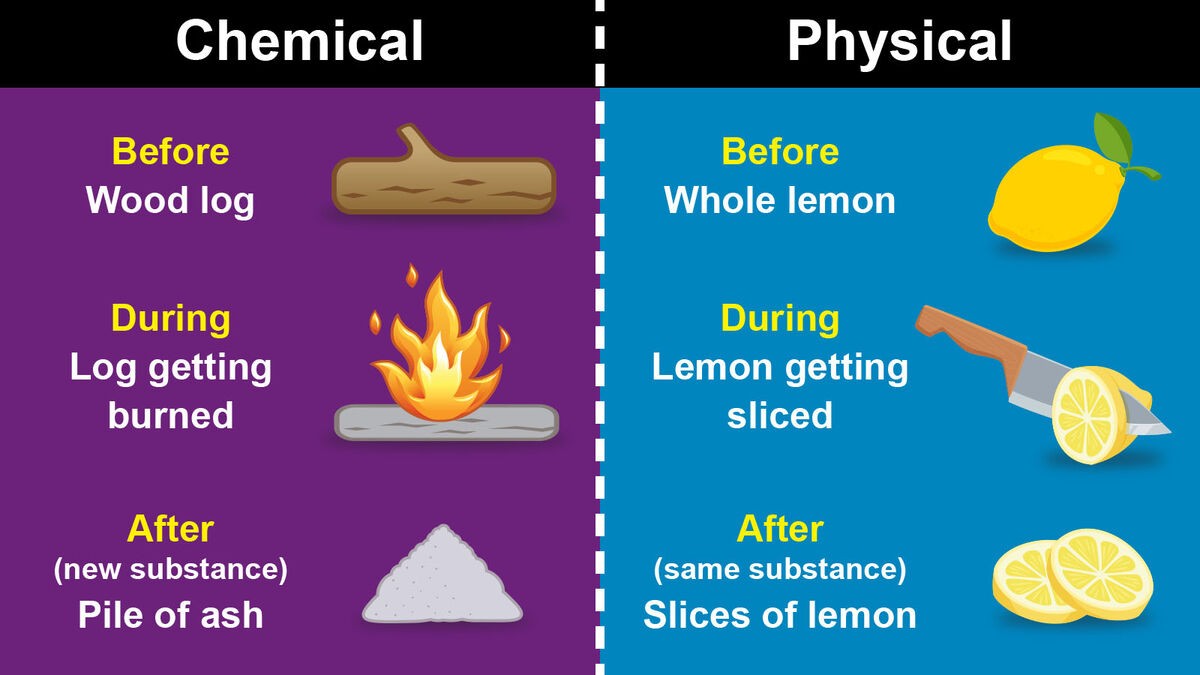 |
Correct Answer is A
Explanation
The diaphragm is a dome-shaped muscle that plays a key role in breathing. It separates the thoracic cavity, which contains the heart and lungs, from the abdominal cavity. When the diaphragm contracts, it moves downward and increases the volume of the thoracic cavity, allowing air to flow into the lungs. When it relaxes, it moves upward and decreases the volume of the thoracic cavity, forcing air out of the lungs.
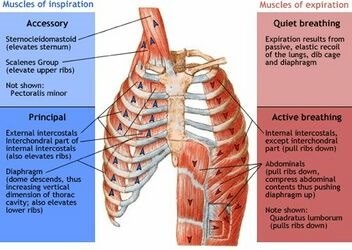 |
Correct Answer is B
Explanation
Ligaments are tough bands of fibrous tissue that connect two bones together in a joint. They provide stability and support to the joint, preventing excessive movement and helping to maintain proper alignment of the bones.
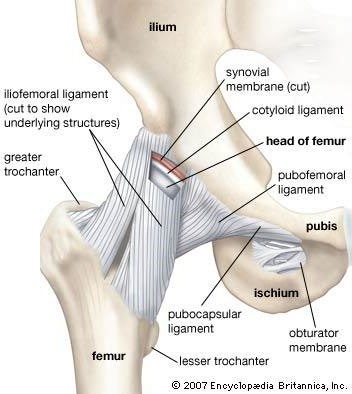
Correct Answer is C
Explanation
One of the main functions of the respiratory system is to facilitate the exchange of gases between the body and the environment. During inhalation, air enters the lungs and oxygen is absorbed into the bloodstream. During exhalation, carbon dioxide is removed from the body and expelled into the environment.
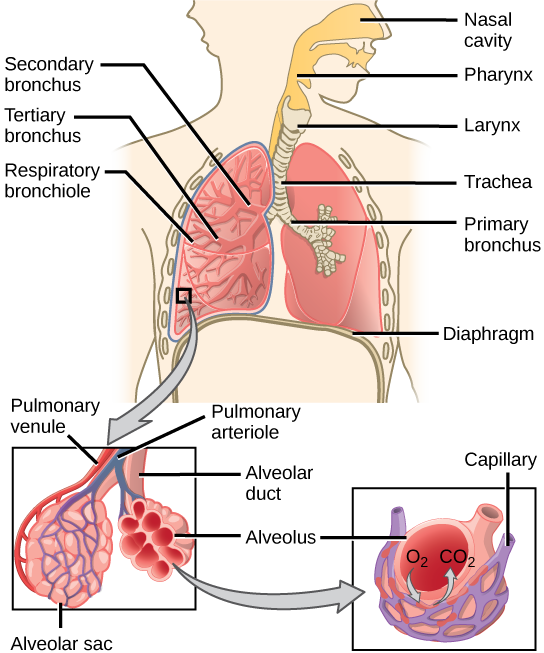 |
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
