Which of the following summarizes a change that takes place as a solid turn to a liquid?
Particles have a decrease in mobility.
Particles become less ordered.
Intermolecular forces between particles become stronger.
Particles move closer together.
Correct Answer : B
: Matter exists in three states: solid, liquid and gases. In solids, the particles are arranged in a uniform pattern and very close to one another, and vibrate within their fixed positions. However, adding more heat to the solid increases the kinetic energy of the particles in solid and slowly turn in liquid state in a process called melting. Particles in liquids are not so close together but can slide past one another and take the shape of the container they are placed. The arrangement of particles of particles declines as the solid turns to liquids. Further addition of heat to a liquid turns it to gaseous state in a process called vaporization. In gaseous state, the particles move randomly and are far apart from one another.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is A
Explanation
As the temperature increase, the liquid turns into a gas. This process is known as vaporization, and in gaseous state the particles become far apart and move in random motion. The change is visually presented below
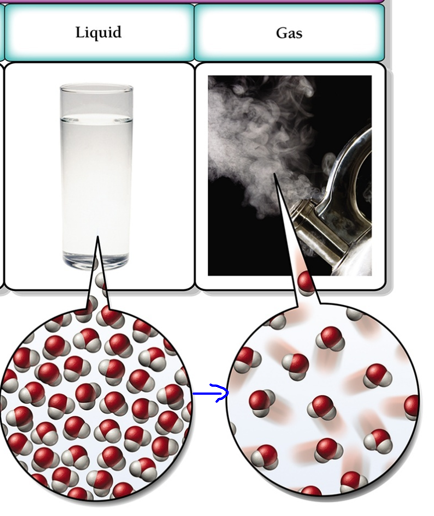
Correct Answer is B
Explanation
: Matter exists in three states: solid, liquid and gases. In solids, the particles are arranged in a uniform pattern and very close to one another, and vibrate within their fixed positions. However, adding more heat to the solid increases the kinetic energy of the particles in solid and slowly turn in liquid state in a process called melting. Particles in liquids are not so close together but can slide past one another and take the shape of the container they are placed. The arrangement of particles of particles declines as the solid turns to liquids. Further addition of heat to a liquid turns it to gaseous state in a process called vaporization. In gaseous state, the particles move randomly and are far apart from one another.
Correct Answer is B
Explanation
phase diagrams are used to indicate physical states, a substance exist in specific conditions of pressure and temperature. Besides, phase diagrams provide information on pressure dependence of the phase-transition temperatures such as melting, sublimation, and boiling points. The figure below shows a typical phase diagram.
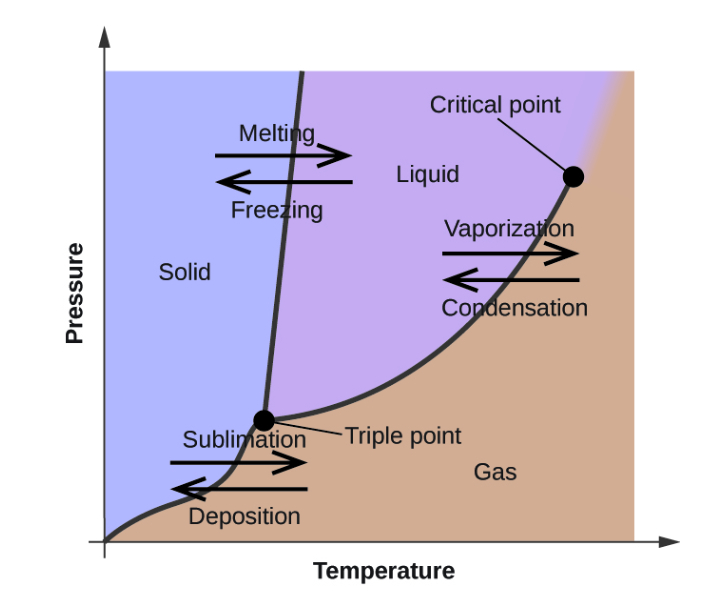
From the above diagram, the point where a substance exists in three states i.e solid, gas, and liquid is known as the triple point.
Correct Answer is A
No explanation
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
