Which of the following is the structure through which blood exits the glomerulus?
Efferent arteriole
Proximal tubule
Distal tubule
Afferent arteriole
Correct Answer : A
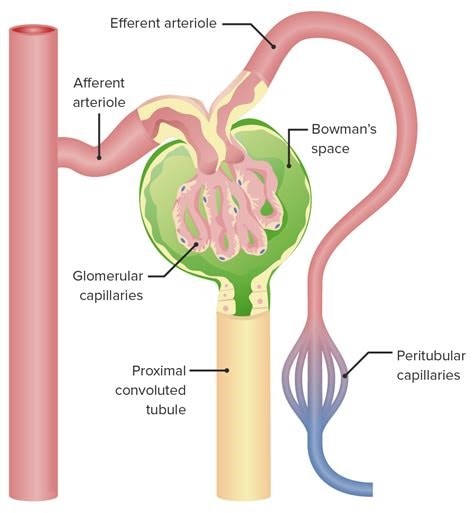
The glomerulus is the main filtering unit of the kidney.
It is formed by a network of small blood vessels (capillaries) enclosed within a sac called the Bowman’s capsule.
The blood supply to the glomerulus is provided via the afferent arteriole.
The blood then flows through the capillary network, where it gets filtered, and then leaves the glomerulus via the efferent arteriole.
Choice B.
Proximal tubule is not correct because it is where the ultrafiltrate collected in the Bowman’s space drains directly into.
Choice C.
Distal tubule is not correct because it is not mentioned in relation to blood exiting the glomerulus.
Choice D.
Afferent arteriole is not correct because it provides blood supply to the glomerulus.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is C
Explanation
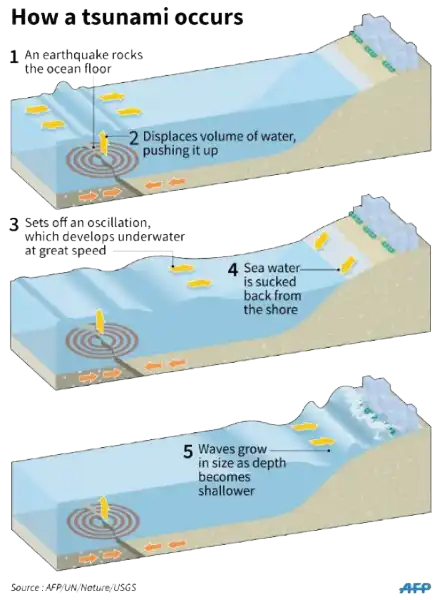
A tsunami is a catastrophic ocean wave that is usually caused by a submarine earthquake.
It can also be caused by an underwater or coastal landslide, the eruption of a volcano, or the impact of a meteor or comet in a body of water.
Choice A is not correct because sunspot activity does not cause tsunamis.
Choice B is not correct because lightning strikes do not cause tsunamis.
Choice D is not correct because flooding does not cause tsunamis.
Correct Answer is A
Explanation
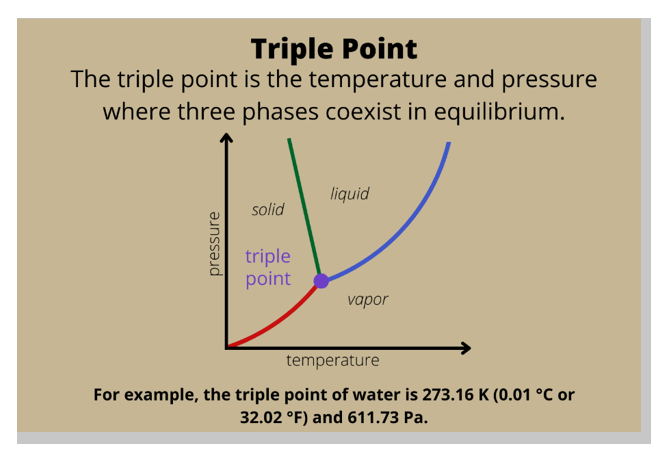
Triple point.
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Correct Answer is C
Explanation
ff.
In this cross, both parents are homozygous recessive for the smooth leaf trait
(ff).
This means that all of their offspring will inherit two copies of the recessive allele (f) and will therefore have smooth leaves.
Choice A.
FF x FF is not correct because both parents are homozygous dominant for the fuzzy leaf trait (FF) and all of their offspring will inherit two copies of the dominant allele (F) and will therefore have fuzzy leaves.
Choice B.
Ff x Ff is not correct because both parents are heterozygous for the leaf trait (Ff) and their offspring can inherit either one dominant allele (F) or one recessive allele (f) from each parent, resulting in a 3:1 ratio of fuzzy to smooth leaves. Choice D.
Ff x ff is not correct because one parent is heterozygous for the leaf trait (Ff) while the other is homozygous recessive (ff), resulting in a 1:1 ratio of fuzzy to smooth leaves in their offspring.
Correct Answer is D
Explanation
Genes are used in the process of DNA sequencing to determine the order of nucleotides in a DNA molecule.
Choice B.
Blood types is not the correct answer because blood types are determined by the presence or absence of specific antigens on the surface of red blood cells and are not directly related to DNA sequencing.
Choice C.
Hormones is not the correct answer because hormones are chemical messengers produced by glands in the body and are not directly involved in DNA sequencing.
Choice D.
Genes is the correct answer because genes are used in the process of DNA sequencing to determine the order of nucleotides in a DNA molecule.
Correct Answer is B
Explanation
Antidiuretic hormone (ADH), also known as vasopressin, is a hormone that helps regulate the amount of water in your body.
It works to control the amount of water your kidneys reabsorb as they filter out waste from your blood.
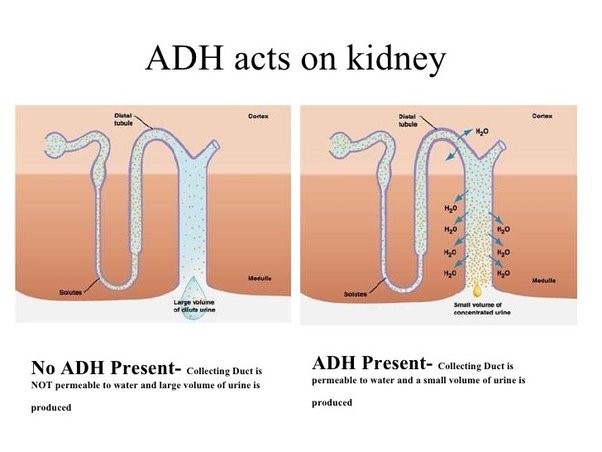
Choice A is not correct because an increase in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice C is not correct because a decrease in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice D is not correct because a decrease in water reabsorption in the collecting duct is not a physiological response caused by the release of antidiuretic hormone.
Correct Answer is D
Explanation
The cytoskeleton of a cell is comprised of protein fibers that provide structural support and help maintain the shape of the cell.
These protein fibers include microfilaments, intermediate filaments, and microtubules.
Choice A.
Carbohydrates is not the correct answer because carbohydrates are a type of macromolecule that provides energy to cells and are not a component of the cytoskeleton.
Choice B.
Nucleic acids is not the correct answer because nucleic acids are macromolecules that store and transmit genetic information and are not a component of the cytoskeleton.
Choice C.
Lipids is not the correct answer because lipids are a type of macromolecule that makes up cell membranes and are not a component of the cytoskeleton.
Correct Answer is C
Explanation
Control.
A control group is a group in an experiment that does not receive the treatment or manipulation being tested and is used as a benchmark to measure how the other tested subjects do.
The control group is used to minimize the effects of all variables except the independent variable.
This allows researchers to determine if changes in the dependent variable are due to the manipulation of the independent variable or if they are due to some other factor.
Choice A.
Responding is not the correct answer because it refers to the dependent variable, which is the variable that is being measured in an experiment.
Choice B.
Manipulated is not the correct answer because it refers to the independent variable, which is the variable that is being manipulated in an experiment.
Choice D.
Variable is not the correct answer because it refers to any factor that can change in an experiment and can include both independent and dependent variables.
Correct Answer is C
Explanation
Plasma B cells.
Antibodies are produced by specialized white blood cells called B lymphocytes (or B cells).
When an antigen binds to the B-cell surface, it stimulates the B cell to divide and mature into a group of identical cells called a clone.
The mature B cells, called plasma cells, secrete millions of antibodies into the bloodstream and lymphatic system.
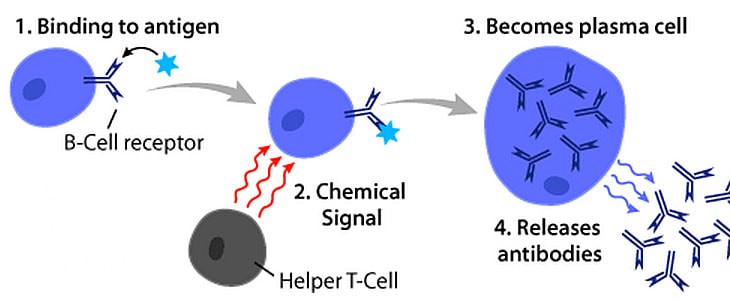
Choice A, Natural killer cells, is not the correct answer because natural killer cells are a type of white blood cell that play a major role in the host-rejection of both tumors and virally infected cells.
Choice B, Cytotoxic T-cells, is not the correct answer because cytotoxic T-cells are a type of white blood cell that kills cancer cells, cells that are infected (particularly with viruses), or cells that are damaged in other ways.
Choice D, Helper T-cells, is not the correct answer because helper T-cells are a type of white blood cell that play an important role in the immune system by helping other white blood cells fight infections.
Correct Answer is D
Explanation
Microtubule organization.
Centrosomes are organelles that serve as the main microtubule-organizing centers for animal cells.
They regulate the movement of microtubules and other cytoskeletal structures, thereby facilitating changes in the shapes of the membranes of animal cells.
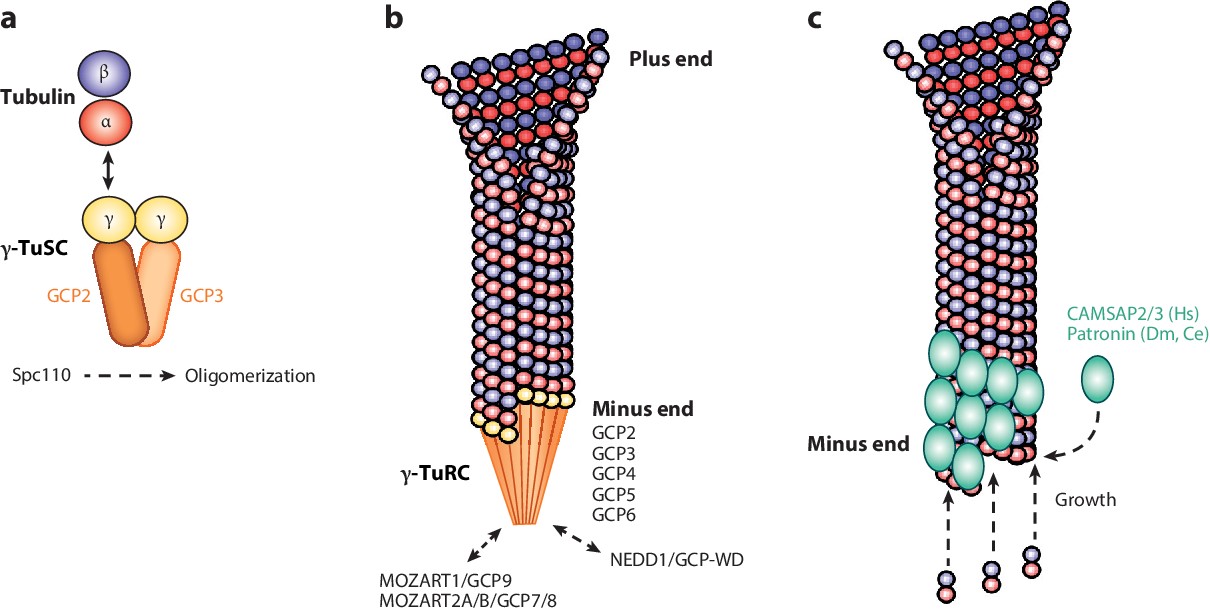
Choice A, Organelle trafficking, is not the correct answer because while centrosomes do play a role in intracellular trafficking during interphase by organizing an astral ray of microtubules, their main function is microtubule organization.
Choice B, Pathogen digestion, is not the correct answer because centrosomes do not play a direct role in pathogen digestion.
Choice C, Cytoplasm formation, is not the correct answer because centrosomes do not play a direct role in cytoplasm formation.
Correct Answer is D
Explanation
The polarity of water molecules explains its solvent abilities for certain substances.
Water is a polar molecule because it has a partial positive charge on one end and a partial negative charge on the other end due to the unequal sharing of electrons between the oxygen and hydrogen atoms.
This polarity allows water to dissolve other polar substances and ionic compounds.
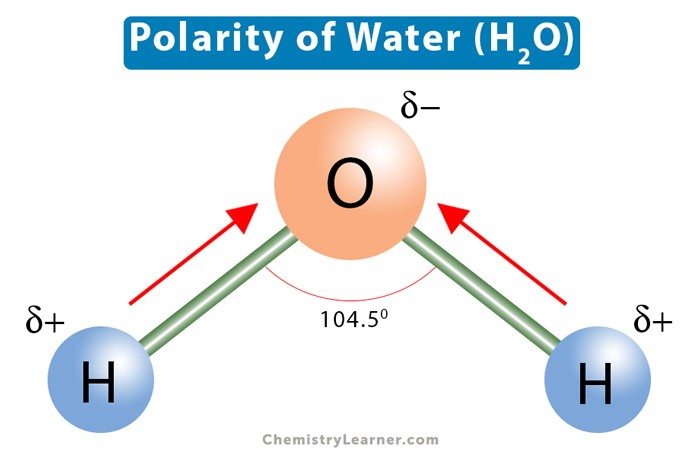
Choice A.
Kinetic energy of liquid water molecules is not the correct answer because kinetic energy refers to the energy of motion and does not directly explain water’s solvent abilities.
Choice B.
High specific heat is not the correct answer because specific heat refers to the amount of heat required to raise the temperature of a substance and does not directly explain water’s solvent abilities.
Choice C.
High surface tension is not the correct answer because surface tension refers to the cohesive forces between liquid molecules and does not directly explain water’s solvent abilities.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
