Which of the following is the number of protons in a lithium atom?
7
3
12
4
Correct Answer : B
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.
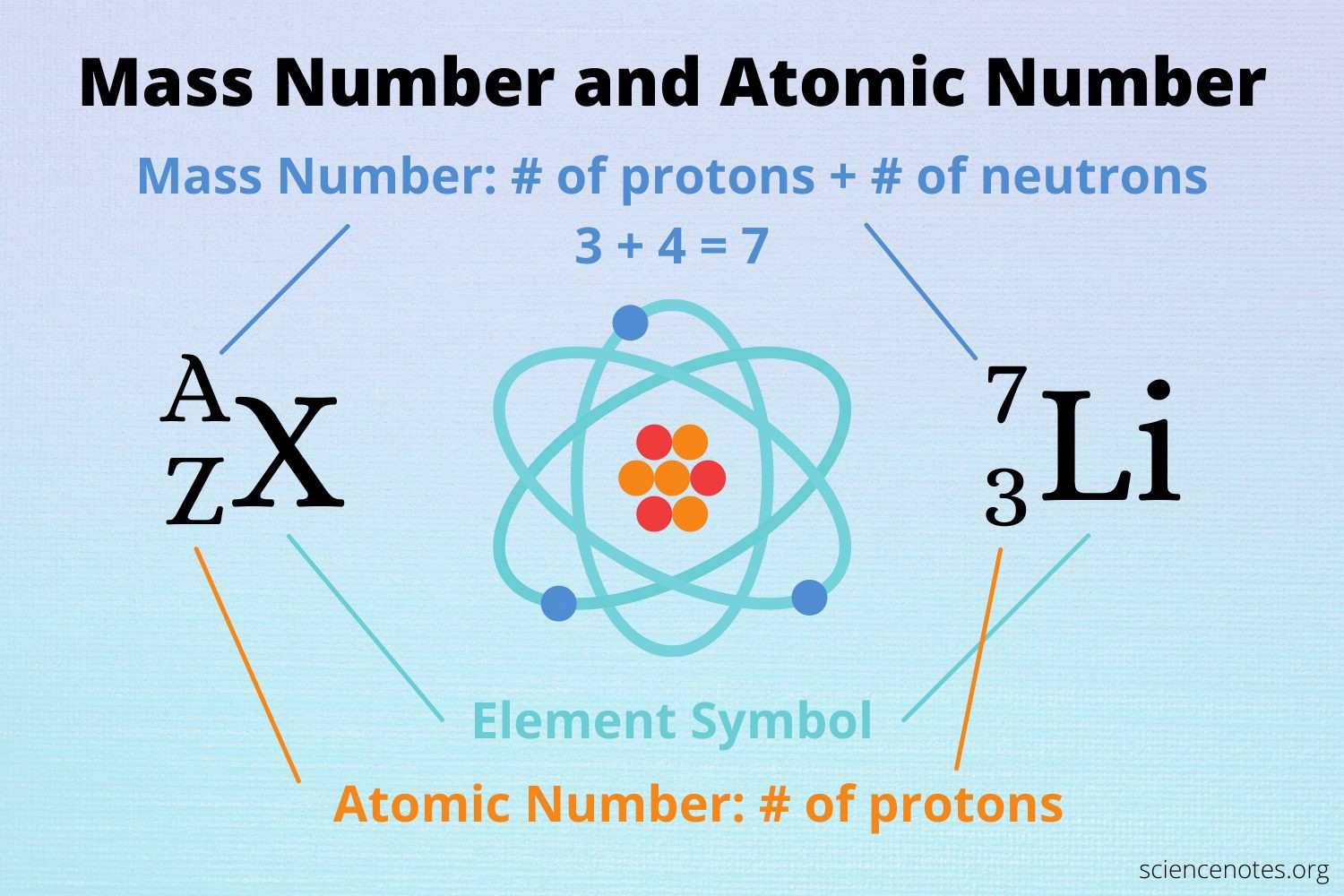
Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is A
Explanation
The approximate threshold value for mammalian neurons is -55 mV.
The threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential.
Most often, the threshold potential is a membrane potential value between –50 and –55 mV
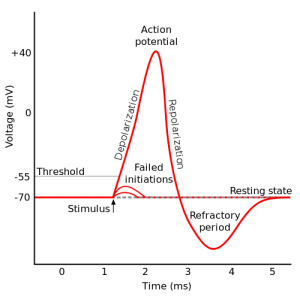
The membrane potential of a neuron is determined by the distribution of ions across the cell membrane.
At rest, the inside of a neuron is more negative than the outside due to the presence of negatively charged proteins and other molecules.
The movement of ions across the cell membrane can change the membrane potential.
For example, when sodium ions enter the cell, they make the inside of the cell more positive (less negative), causing depolarization.
Choice B is incorrect because -80 mV is below the typical threshold value for mammalian neurons.
Choice C is incorrect because +35 mV is above the typical threshold value for mammalian neurons.
Choice D is incorrect because 0 mV is above the typical threshold value for mammalian neurons.
Correct Answer is D
Explanation
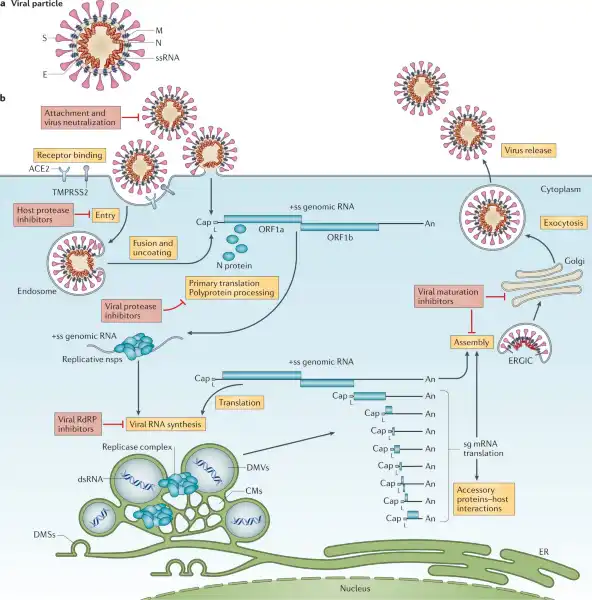
Viruses.
Viruses lack essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are singlecelled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Correct Answer is C
Explanation
Other scientists can validate or disprove the findings.
It is important for new scientific findings to be published so that other scientists can review the research and either validate or disprove the findings.
This process of peer-review helps to ensure the accuracy and reliability of scientific research.
Choice A.
Scientists will get paid if their findings are published is not correct because while some scientists may receive funding or grants for their research, the primary goal of publishing scientific findings is not for financial gain.
Choice B.
Publishing findings will help scientists become more biased is not correct because the goal of publishing scientific findings is to share information and promote transparency, not to promote bias.
Choice D.
This prevents other scientists from performing similar tests is not correct because publishing scientific findings allows other scientists to build upon the research and perform further tests to validate or disprove the findings.
Correct Answer is A
Explanation
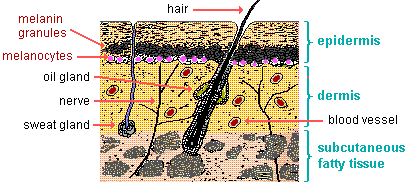
Melanin.
Melanin is a pigment produced by cells called melanocytes in the skin.
It protects the skin from ultraviolet (UV) radiation by absorbing and dissipating over 99.9% of absorbed UV radiation.
This helps to prevent DNA damage and other adverse effects of UV radiation on the skin.
Choice B.
Perspiration is not correct because it is a fluid produced by sweat glands in the skin that helps to regulate body temperature, but it does not protect the skin from UV radiation.
Choice C.
Sebum is not correct because it is an oily substance produced by sebaceous glands in the skin that helps to lubricate and protect the skin, but it does not protect the skin from UV radiation.
Choice D.
Keratin is not correct because it is a fibrous protein that provides strength and durability to the skin, hair and nails, but it does not protect the skin from UV radiation.
Correct Answer is A
Explanation
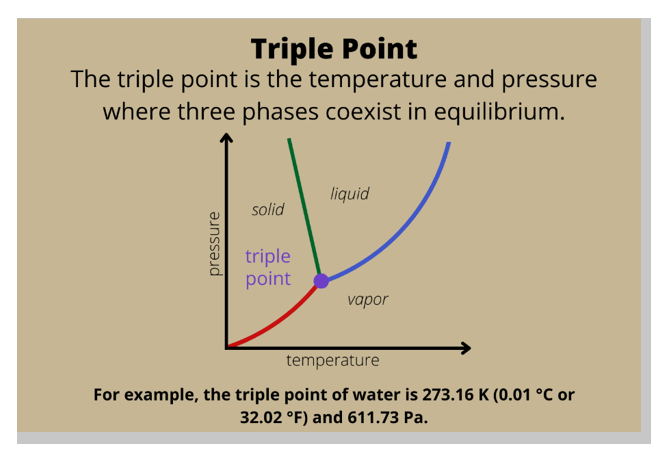
Triple point.
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Correct Answer is B
Explanation
The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.

Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
Correct Answer is D
Explanation
The pleura is a double-layered serous membrane that covers each lung and lines the thoracic cage
The pleura is a vital part of the respiratory tract.
Its role is to cushion the lung and reduce any friction that may develop between the lung, rib cage, and chest cavity.
Each pleura (there are two) consists of a two-layered membrane that covers each lung.
The layers are separated by a small amount of viscous (thick) lubricant known as pleural fluid.
The pleura is comprised of two distinct layers: the visceral pleura and the parietal pleura.
The visceral pleura is the thin, slippery membrane that covers the surface of the lungs and dips into the areas separating the different lobes of the lungs (called the hilum).
Correct Answer is D
Explanation
Genes that regulate cell division are found in some viruses.
When viruses cause an infection, they spread their DNA, affecting healthy cells’ genetic makeup and potentially causing them to turn into cancer.
For instance, HPV infections cause the virus’ DNA to combine with the host’s DNA, disrupting the normal function of cells.
Choice A is not correct because cancerous and normal cells sharing genetic sequences does not support the hypothesis that viruses can cause cancer.
Choice B is not correct because cellular DNA having sequences related to viral sequences does not support the hypothesis that viruses can cause cancer.
Choice C is not correct because viruses and cancer cells both replicating rapidly does not support the hypothesis that viruses can cause cancer.
Correct Answer is D
Explanation
Genes are used in the process of DNA sequencing to determine the order of nucleotides in a DNA molecule.
Choice B.
Blood types is not the correct answer because blood types are determined by the presence or absence of specific antigens on the surface of red blood cells and are not directly related to DNA sequencing.
Choice C.
Hormones is not the correct answer because hormones are chemical messengers produced by glands in the body and are not directly involved in DNA sequencing.
Choice D.
Genes is the correct answer because genes are used in the process of DNA sequencing to determine the order of nucleotides in a DNA molecule.
Correct Answer is B
Explanation
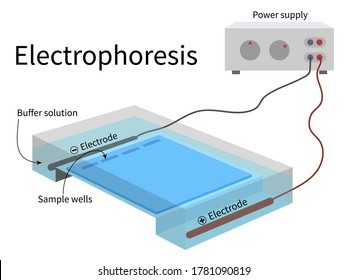
Electrophoresis is the most useful laboratory method for separating genomic DNA fragments by size.
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
