Which of the following is the atomic number of an atom that has 12 protons and 12 neutrons?
144
12
24
1
Correct Answer : B
elements are arranged on the periodic table based on their atomic number. This identity is critical in evaluating the chemical families of elements in chemistry. Atoms are made of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus while electrons are found outside the nucleus of the atom. It is the number of protons of an atom that gives its atomic number. Therefore, an atom with 12 protons has an atomic number of 12.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
We find the number of neutrons as follows
Number of neutrons
= mass number – atomic number
= 207 – 82
= 125 neutrons
Correct Answer is B
Explanation
elements are arranged on the periodic table based on their atomic number. This identity is critical in evaluating the chemical families of elements in chemistry. Atoms are made of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus while electrons are found outside the nucleus of the atom. It is the number of protons of an atom that gives its atomic number. Therefore, an atom with 12 protons has an atomic number of 12.
Correct Answer is A
Explanation
The atom is identified by the atomic number in the periodic table. The atomic number is equal to the number of protons and mass number is the sum of the number of protons and neutrons in the nucleus of an atom. Thus, to find how many neutrons are in lithium atom we use the formula below.
Mass number = number of protons + number of neutrons.
Letting n be number of neutrons in the lithium atom, we have
7=3+n
Rearranging the above equation
3+n=7
n=7-3
n=4
Thus, lithium atom has 4 neutrons.
Or (Other explanation)
To find the number of neutrons in a lithium atom, you subtract the atomic number (number of protons) from the mass number.
Given:
Atomic number of lithium (Z) = 3
Mass number (A) = 7
Number of neutrons (N) = Mass number (A) - Atomic number (Z)
So,
Number of neutrons = 7 - 3 = 4
Therefore, the correct answer is4
Correct Answer is C
Explanation
The Lewis structure of nitrogen gas is shown below. Triple bonds are stronger than double or single bonds. Therefore, we can infer that the triple bond between the two nitrogen atoms makes nitrogen gas more stable.
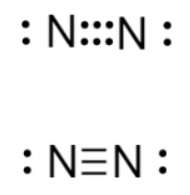
Correct Answer is D
No explanation
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
