Which of the following is correct regarding the pH scale?
A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4
A substance with a pH of 3 is two times more acidic than a substance with a pH of 4
A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4
A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4
Correct Answer : C
The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution.
A solution with a pH of 7 is neutral, while a solution with a pH less than 7 is acidic and a solution with a pH greater than 7 is alkaline.
Because the pH scale is logarithmic, each whole number change in pH represents a tenfold change in acidity or alkalinity.
Therefore, a substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4.
Choice A.
A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline and also incorrectly states the magnitude of the difference in acidity or alkalinity.
Choice B.
A substance with a pH of 3 is two times more acidic than a substance with a pH of 4 is not correct because it correctly states that the substance with a lower pH is more acidic but incorrectly states the magnitude of the difference in acidity.
Choice D.
A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is D
Explanation
Viruses.
Viruses lack essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
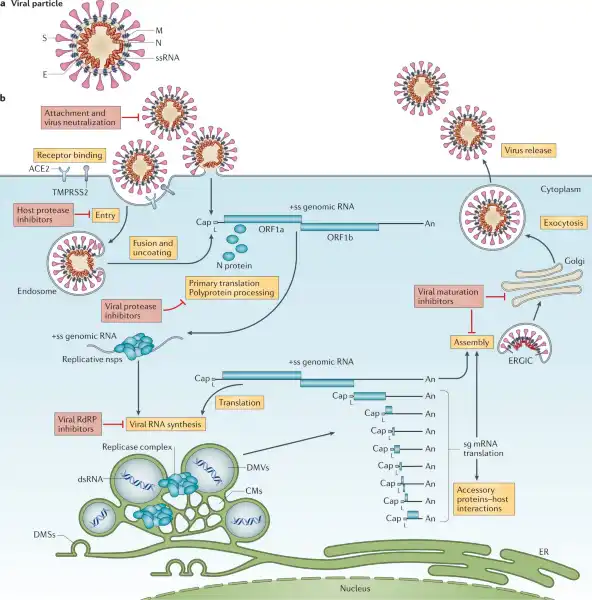
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are singlecelled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Correct Answer is A
Explanation
The glomerulus is the main filtering unit of the kidney.
It is formed by a network of small blood vessels (capillaries) enclosed within a sac called the Bowman’s capsule.
The blood supply to the glomerulus is provided via the afferent arteriole.
The blood then flows through the capillary network, where it gets filtered, and then leaves the glomerulus via the efferent arteriole.
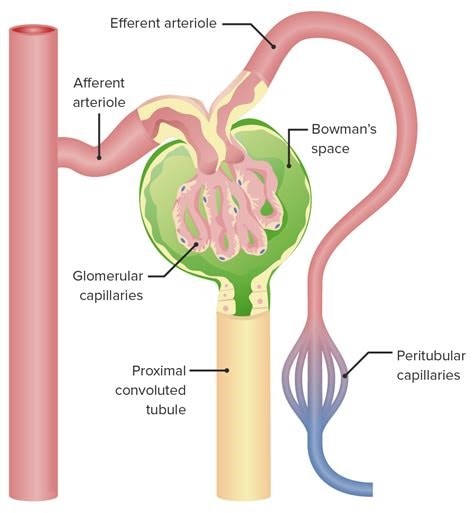
Choice B.
Proximal tubule is not correct because it is where the ultrafiltrate collected in the Bowman’s space drains directly into.
Choice C.
Distal tubule is not correct because it is not mentioned in relation to blood exiting the glomerulus.
Choice D.
Afferent arteriole is not correct because it provides blood supply to the glomerulus.
Correct Answer is C
Explanation
Carbonic acid.
In the human body, maintaining the pH of the blood within a narrow range is critical for proper physiological functioning.
One of the buffering systems that helps to regulate blood pH involves the conversion of carbon dioxide (CO2) and water (H2O) into carbonic acid (H2CO3), which then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-).
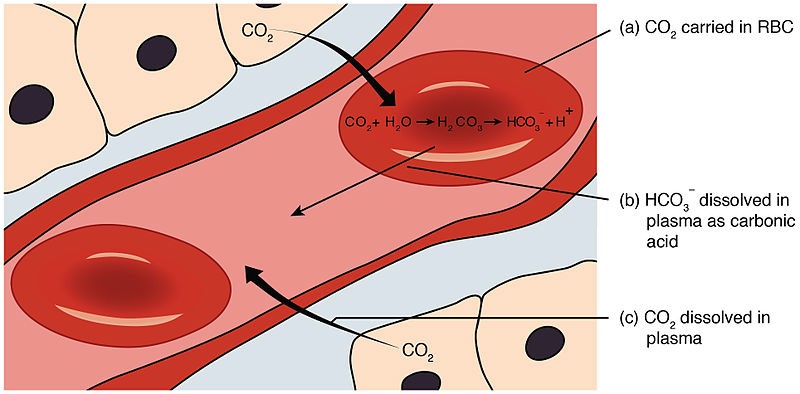
Carbonic acid (H2CO3) is responsible for donating H+ ions to act as a buffer when blood pH rises.
When blood pH rises (becomes more alkaline), carbonic acid dissociates, and the H+ ions combine with bicarbonate ions to form more carbonic acid.
This helps to remove excess H+ ions from the blood and prevent the pH from rising too much.
Option A, carbon dioxide, is involved in the buffering system through its conversion to carbonic acid.
However, it does not directly donate H+ ions to act as a buffer when blood pH rises.
Option B, carbon monoxide, is a toxic gas that binds to hemoglobin in red blood cells, preventing them from carrying oxygen.
It is not involved in the buffering system and does not donate H+ ions.
Option D, oxygen, is carried by hemoglobin in red blood cells and is essential for respiration.
It is not involved in the buffering system and does not donate H+ ions.
Correct Answer is B
Explanation
Reverse transcriptase, an enzyme encoded by the virus.
Reverse transcriptase is a virus-specific enzyme that transcribes an RNA template to DNA1.
This allows the AIDS virus, which contains RNA, to insert viral DNA into the DNA of a host cell after the AIDS virus enters the cell.
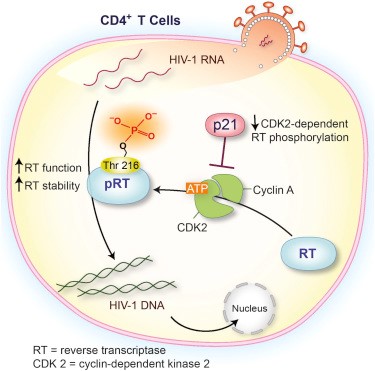
Choice A, The phospholipids found on the envelope of the virus, is not the correct answer because phospholipids are a major component of cell membranes and do not play a direct role in inserting viral DNA into the DNA of a host cell.
Choice C, Receptor proteins located on the surface of the virus, is not the correct answer because receptor proteins located on the surface of the virus play a role in attachment and fusion of HIV virons to host cells, but do not play a direct role in inserting viral DNA into the DNA of a host cell.
Choice D, The protein that makes up the capsid of the virus, is not the correct answer because capsid is the outer protein shell of a virus and does not play a direct role in inserting viral DNA into the DNA of a host cell.
Correct Answer is D
Explanation
The pulmonary veins are the vessels that carry oxygenated blood from the lungs to the left atrium of the heart.
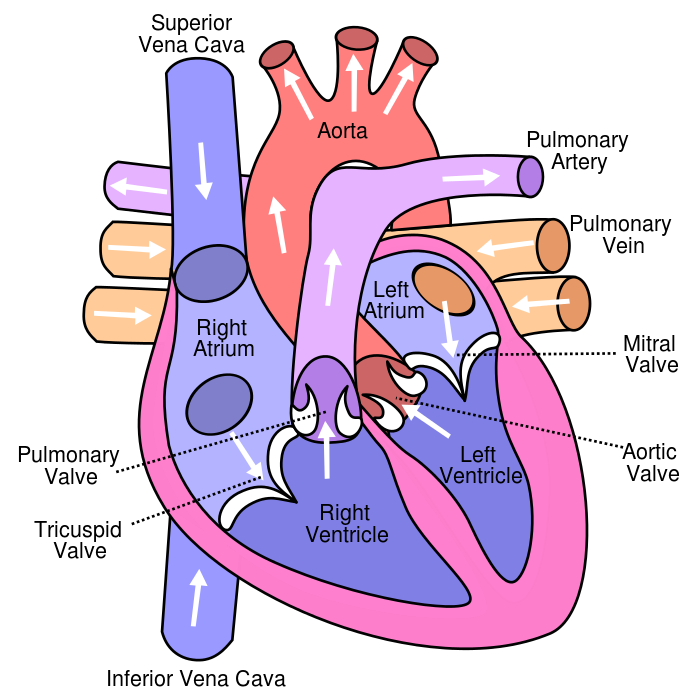
Choice A is not correct because the superior vena cava carries deoxygenated blood from the upper body to the right atrium of the heart.
Choice B is not correct because the inferior vena cava carries deoxygenated blood from the lower body to the right atrium of the heart.
Choice C is not correct because the pulmonary artery carries deoxygenated blood from the right ventricle of the heart to the lungs.
Correct Answer is B
Explanation
Antidiuretic hormone (ADH), also known as vasopressin, is a hormone that helps regulate the amount of water in your body.
It works to control the amount of water your kidneys reabsorb as they filter out waste from your blood.
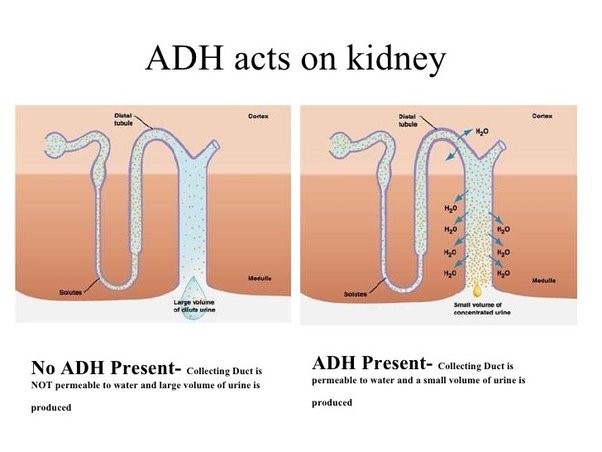
Choice A is not correct because an increase in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice C is not correct because a decrease in the concentration of calcium in the glomerulus is not a physiological response caused by the release of antidiuretic hormone.
Choice D is not correct because a decrease in water reabsorption in the collecting duct is not a physiological response caused by the release of antidiuretic hormone.
Correct Answer is C
Explanation
A tsunami is a catastrophic ocean wave that is usually caused by a submarine earthquake.
It can also be caused by an underwater or coastal landslide, the eruption of a volcano, or the impact of a meteor or comet in a body of water.
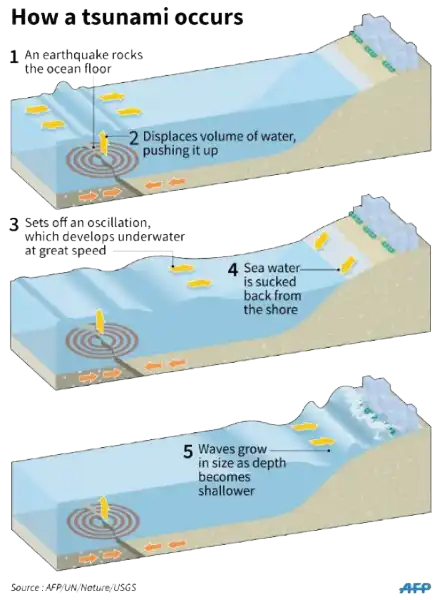
Choice A is not correct because sunspot activity does not cause tsunamis.
Choice B is not correct because lightning strikes do not cause tsunamis.
Choice D is not correct because flooding does not cause tsunamis.
Correct Answer is B
Explanation
The atomic number of an atom is equal to the number of protons in its nucleus.
In this case, the atom has 12 protons, so its atomic number is 12.
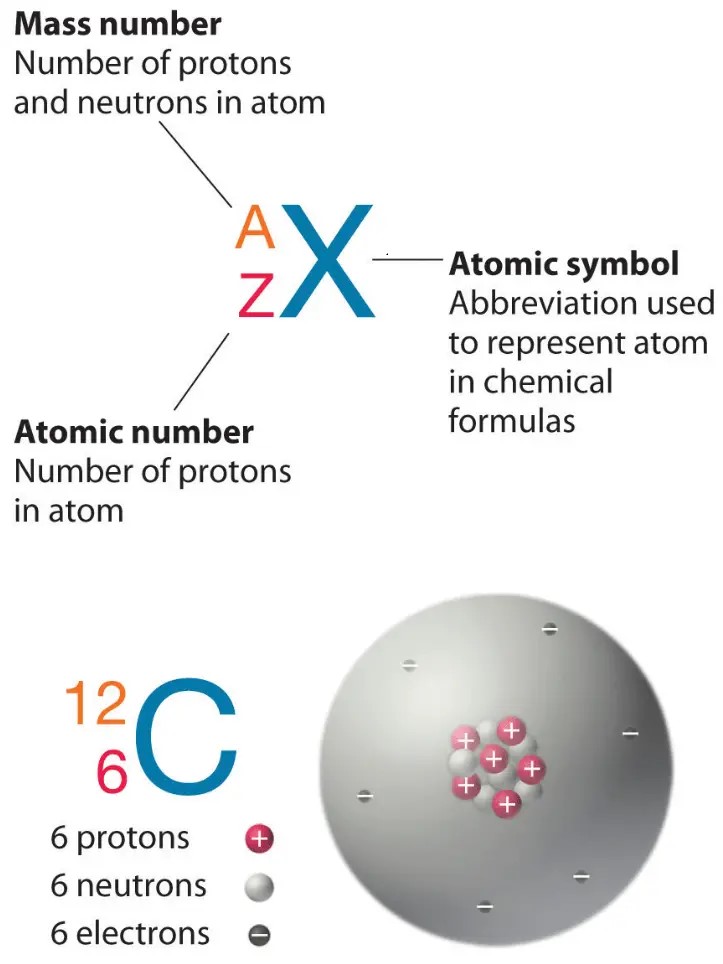
Choice A, 24, is not the correct answer because it represents the sum of the number of protons and neutrons in the atom’s nucleus, which is known as the mass number.
Choice C, 1, is not the correct answer because it does not represent the number of protons in the atom’s nucleus.
Choice D, 144, is not the correct answer because it represents the square of the mass number and does not represent any property of the atom.
Correct Answer is C
Explanation
Ovulation is the process in which an ovarian follicle matures and releases a reproductive egg.
During ovulation, a mature egg is released from the female ovary, enabling it to be fertilized by male sperm cells 1.
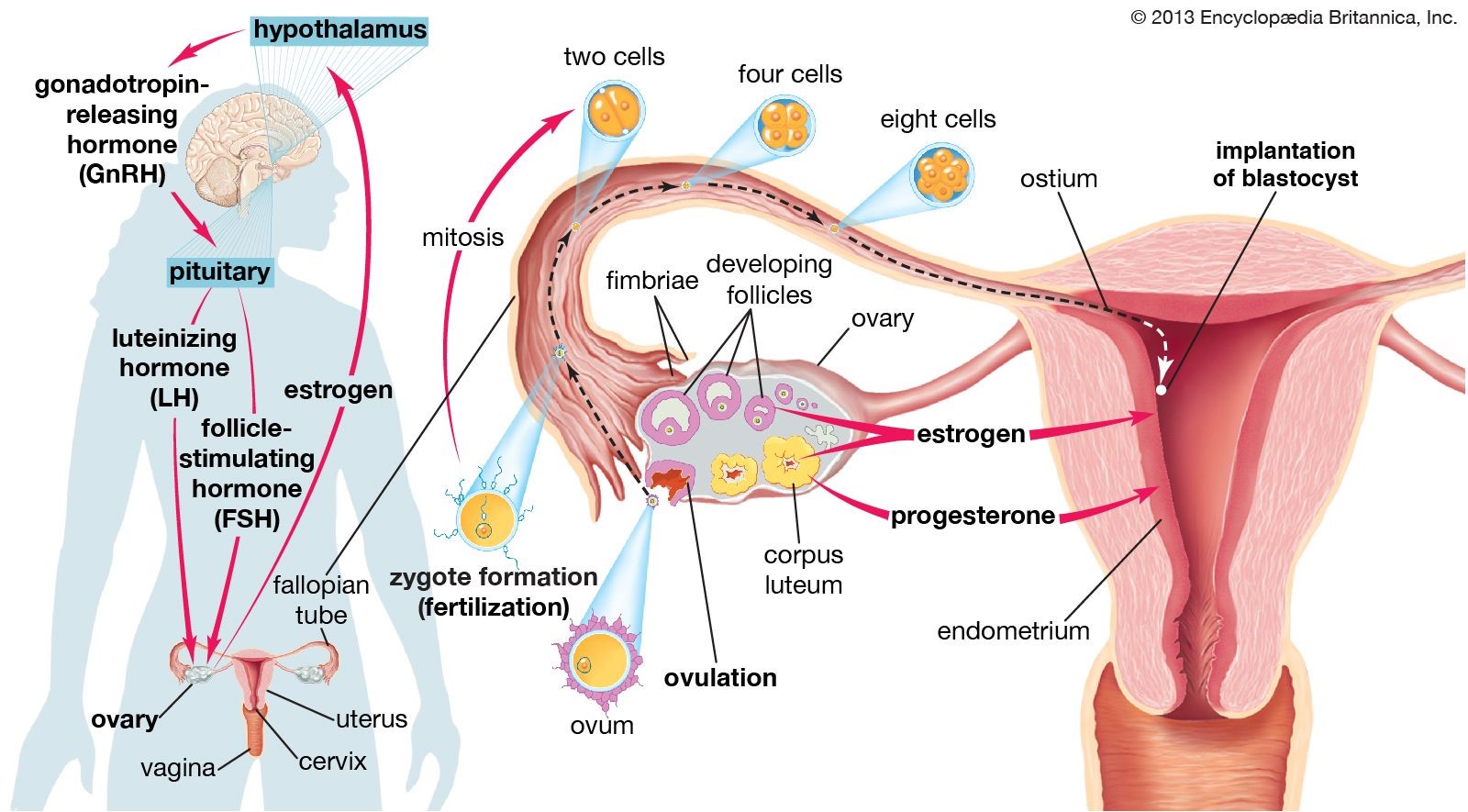
Choice A is incorrect because menstruation is the process of shedding the uterine lining, which occurs when an egg is not fertilized.
Choice B is incorrect because fertilization is the process of a sperm cell joining with an egg cell to form a zygote.
Choice D is incorrect because oogenesis is the process of forming female gametes (eggs) in the ovaries.
Correct Answer is A
Explanation
The approximate threshold value for mammalian neurons is -55 mV.
The threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential.
Most often, the threshold potential is a membrane potential value between –50 and –55 mV
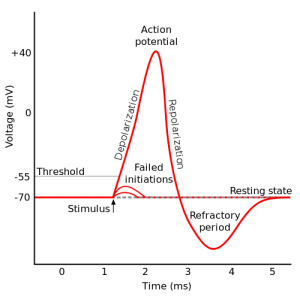
The membrane potential of a neuron is determined by the distribution of ions across the cell membrane.
At rest, the inside of a neuron is more negative than the outside due to the presence of negatively charged proteins and other molecules.
The movement of ions across the cell membrane can change the membrane potential.
For example, when sodium ions enter the cell, they make the inside of the cell more positive (less negative), causing depolarization.
Choice B is incorrect because -80 mV is below the typical threshold value for mammalian neurons.
Choice C is incorrect because +35 mV is above the typical threshold value for mammalian neurons.
Choice D is incorrect because 0 mV is above the typical threshold value for mammalian neurons.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
