Which of the following indicates the function of sodium bicarbonate secreted by the pancreas?
Sodium bicarbonate is a protease that digests carbohydrates.
Sodium bicarbonate stimulates the pyloric sphincter.
Sodium bicarbonate inhibits peristalsis.
Sodium bicarbonate neutralizes the acidity of chyme.
Correct Answer : D
Sodium bicarbonate neutralizes the acidity of chyme.
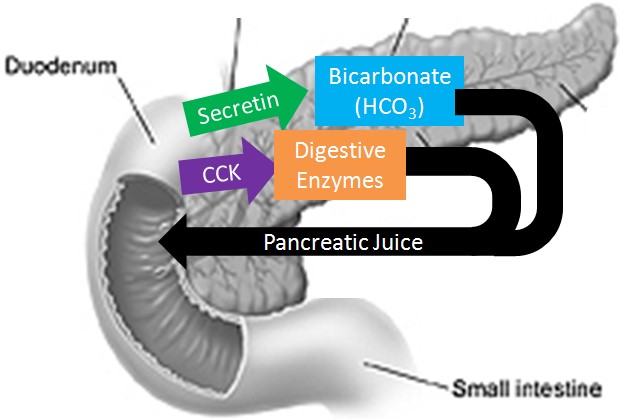
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.
Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
The atomic number of an atom is equal to the number of protons in its nucleus.
In this case, the atom has 12 protons, so its atomic number is 12.
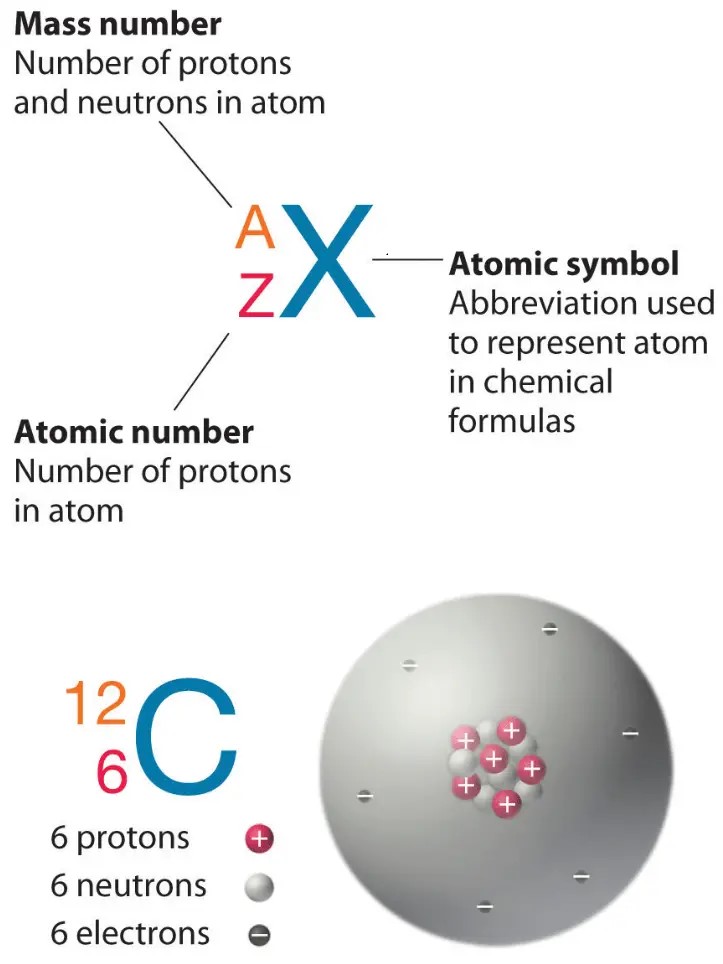
Choice A, 24, is not the correct answer because it represents the sum of the number of protons and neutrons in the atom’s nucleus, which is known as the mass number.
Choice C, 1, is not the correct answer because it does not represent the number of protons in the atom’s nucleus.
Choice D, 144, is not the correct answer because it represents the square of the mass number and does not represent any property of the atom.
Correct Answer is C
Explanation
The best reason for the prolonged preservation of the body is that it was frozen in the cold temperature of the Alps shortly after he died and remained frozen until it was found.
Freezing can preserve a body by slowing down or stopping the decomposition process.
Choice A is not correct because the food that the person ate would not have contained toxins that killed the bacteria that would have otherwise destroyed the body.
Choice B is not correct because the arrow wound would not have caused blood to flow out of the body in a way that would have cleared enzymes that break down tissue from the body.
Choice D is not correct because ultraviolet rays at high altitude would not have caused all of the body’s molecules to be preserved.
Correct Answer is D
Explanation
Viruses.
Viruses lack essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are singlecelled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Correct Answer is B
Explanation
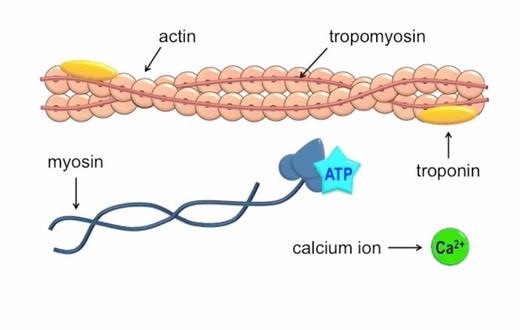
Calcium ions play a crucial role in initiating muscle contraction.
When a muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm.
Some of this calcium attaches to troponin, which causes it to change shape.
This shape change exposes binding sites for myosin on the actin filaments.
Myosin’s binding to actin causes crossbridge formation, and contraction of the muscle begins.
The other ions mentioned in the question do not have this specific role in muscle contraction.
Potassium ions are important for maintaining the resting membrane potential of cells, but they do not bind to the troponin complex.
Phosphorus ions are important for energy metabolism, but they do not bind to the troponin complex.
Sodium ions are important for generating action potentials, but they do not bind to the troponin complex.
Correct Answer is A
Explanation
Urea is a substance that is excreted by sweat glands in response to the breakdown of proteins and the formation of ammonia.
When proteins are broken down, they produce ammonia, which is a highly toxic compound for the body.
Ammonia is then converted into urea and released out of the body through sweat glands.
Choice B.
Sebum is not correct because it is an oily substance secreted by sebaceous glands to lubricate and protect the skin, but it is not related to the breakdown of proteins or the formation of ammonia.
Choice C.
Water is not correct because while it is a component of sweat, it is not specifically related to the breakdown of proteins or the formation of ammonia.
Choice D.
Lysozymes are not correct because they are enzymes found in tears, saliva and other body fluids that have antibacterial properties, but they are not related to the breakdown of proteins or the formation of ammonia.
Correct Answer is C
Explanation
The hypothalamus is a region of the brain that synthesizes antidiuretic hormone (ADH), also known as vasopressin.
ADH is then transported to the posterior pituitary gland via neurohypophysial capillaries, where it is stored until it is ready to be secreted into the circulation.
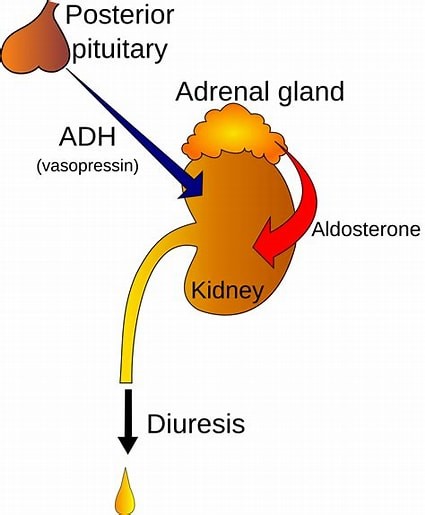
Choice A.
Pineal gland is not correct because it is a small endocrine gland located in the brain that secretes the hormone melatonin, which regulates sleep-wake cycles, but it does not synthesize ADH.
Choice B.
Thymus is not correct because it is a gland located in the chest that produces hormones involved in immune system development, but it does not synthesize ADH.
Choice D.
Pancreas is not correct because it is a gland located behind the stomach that secretes hormones such as insulin and glucagon, which regulate blood sugar levels, but it does not synthesize ADH.
Correct Answer is D
Explanation
Sodium bicarbonate neutralizes the acidity of chyme.
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.

Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
Correct Answer is D
Explanation
Triple covalent bonds.
Nitrogen gas (N2) is an extremely stable molecule because it consists of two nitrogen atoms bonded together by a triple covalent bond.
A covalent bond is a type of chemical bond where atoms share electrons to form a molecule.
In a triple covalent bond, three pairs of electrons are shared between the two atoms, resulting in a very strong bond that makes the molecule extremely stable.
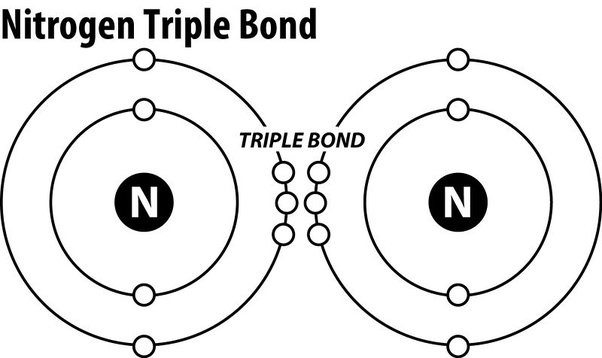
Choice A.
Ionic bonds is not correct because ionic bonds involve the transfer of electrons from one atom to another to form ions, which are then attracted to each other due to their opposite charges.
Nitrogen gas does not contain ions and is not held together by ionic bonds.
Choice B.
Hydrogen bonds is not correct because hydrogen bonds are weak electrostatic attractions between molecules that contain hydrogen atoms bonded to highly electronegative atoms such as oxygen or nitrogen.
Nitrogen gas does not contain hydrogen atoms and is not held together by hydrogen bonds.
Choice C.
Resonance bonds is not correct because resonance refers to the delocalization of electrons in a molecule where multiple Lewis structures can be drawn to represent the molecule.
Nitrogen gas has a single Lewis structure and does not exhibit resonance.
Correct Answer is C
Explanation
ff.
In this cross, both parents are homozygous recessive for the smooth leaf trait
(ff).
This means that all of their offspring will inherit two copies of the recessive allele (f) and will therefore have smooth leaves.
Choice A.
FF x FF is not correct because both parents are homozygous dominant for the fuzzy leaf trait (FF) and all of their offspring will inherit two copies of the dominant allele (F) and will therefore have fuzzy leaves.
Choice B.
Ff x Ff is not correct because both parents are heterozygous for the leaf trait (Ff) and their offspring can inherit either one dominant allele (F) or one recessive allele (f) from each parent, resulting in a 3:1 ratio of fuzzy to smooth leaves. Choice D.
Ff x ff is not correct because one parent is heterozygous for the leaf trait (Ff) while the other is homozygous recessive (ff), resulting in a 1:1 ratio of fuzzy to smooth leaves in their offspring.
Correct Answer is C
Explanation
The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution.
A solution with a pH of 7 is neutral, while a solution with a pH less than 7 is acidic and a solution with a pH greater than 7 is alkaline.
Because the pH scale is logarithmic, each whole number change in pH represents a tenfold change in acidity or alkalinity.
Therefore, a substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4.
Choice A.
A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline and also incorrectly states the magnitude of the difference in acidity or alkalinity.
Choice B.
A substance with a pH of 3 is two times more acidic than a substance with a pH of 4 is not correct because it correctly states that the substance with a lower pH is more acidic but incorrectly states the magnitude of the difference in acidity.
Choice D.
A substance with a pH of 3 is 10 times more alkaline than a substance with a pH of 4 is not correct because it incorrectly states that the substance with a lower pH is more alkaline.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
