Which of the following determines the strength of an acidic solution?
Litmus paper that turns red
Litmus paper that turns blue
Measured pH value equal to 7
Measured pH value less than 7
Correct Answer : D
Both litmus paper and a pH scale can be used to indicate whether a solution is acidic. However, a pH scale can also determine the strength of an acid.
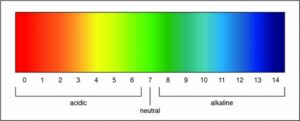
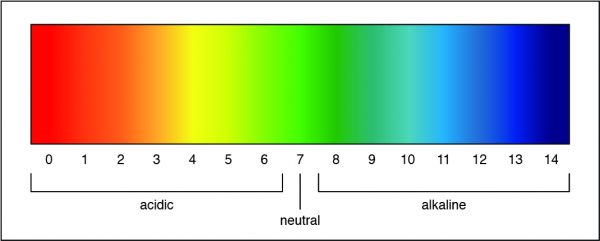
Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is D
Explanation
The autonomic nervous systemis responsible for activities that arenonvoluntaryand under unconscious control. This system controls glands and the smooth muscles of internal organs, heart rate, breathing, and digestion. The autonomic nervous system is further divided into the following:
- Sympathetic nervous system: The sympathetic nervous system focuses on emergency situations by preparing the body forfight or flight. (Sympathetic = Stress)
- Parasympathetic nervous system: The parasympathetic nervous system controls involuntary processes unrelated to emergencies. This system deals with “rest or digest” activities. (Parasympathetic = Peace)
Thesomatic nervous systemprimarily controlsvoluntaryactivities such as walking and riding a bicycle. Thus, this system sends information to the CNS and motor nerve fibers that are attached to skeletal muscle.
Correct Answer is A
Explanation
The protein disc that holds two sister chromatids together is what collectively makes a chromosome. A gene is a segment of DNA, deoxyribonucleic acid, which transmits information from parent to offspring. A single molecule of DNA has thousands of genes. A chromosome is a rod-shaped structure that forms when a single DNA molecule and its associated proteins coil tightly before cell division.
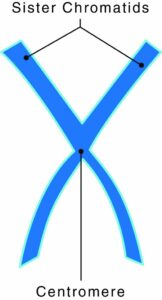
Chromosomes have two components:
- Chromatids: two copies of each chromosome
- Centromeres: protein discs that attach the chromatids together
Human cells have 23 sets of different chromosomes. The two copies of each chromosome are called homologous chromosomes, or homologues. An offspring receives one homologue from each parent. When a cell contains two homologues of each chromosome, it is termed diploid (2n). A haploid (n) cell contains only one homologue of each chromosome. The only haploid cells humans have are the sperm and eggs cells known as gametes.
Correct Answer is D
Explanation
Once the food has been masticated in the oral cavity (mouth), it is then swallowed and travels back into the pharynx down into the esophagus, which leads into the stomach.
Correct Answer is B
Explanation
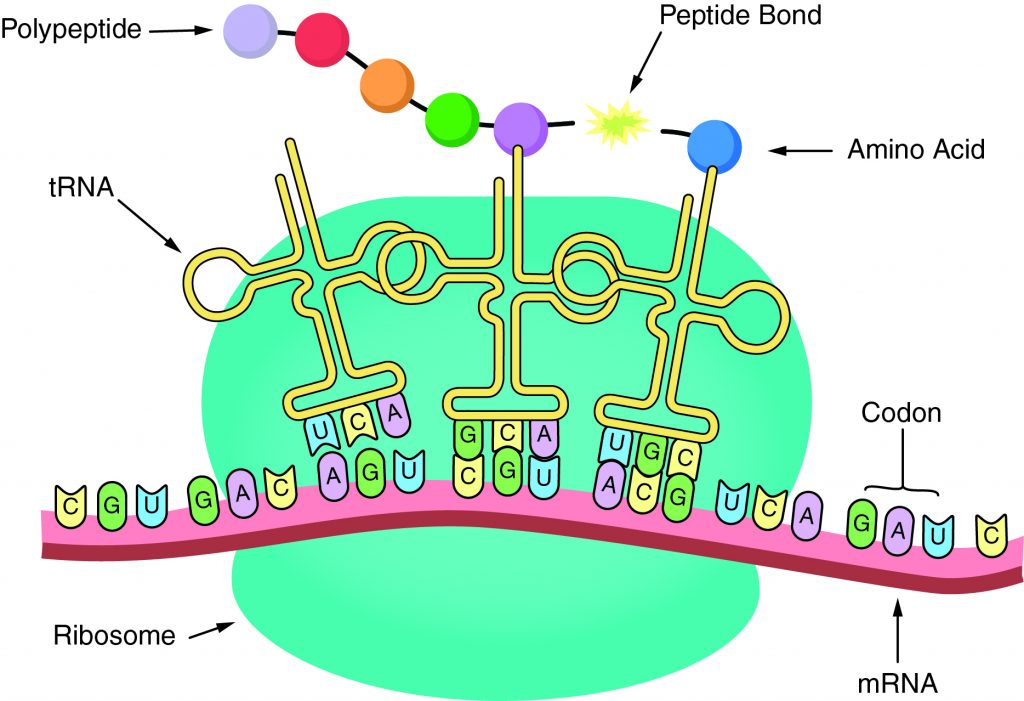
The sequence of amino acids in a gene determines the primary structure of a protein. The components necessary fortranslationare located in the cytoplasm. Translation is the making of proteins by mRNA binding to a ribosome with the start codon that initiates the production of amino acids. Apeptide bondforms and connects the amino acids together. The sequence of amino acids determines the protein’s structure, which determines its function.
Correct Answer is A
Explanation
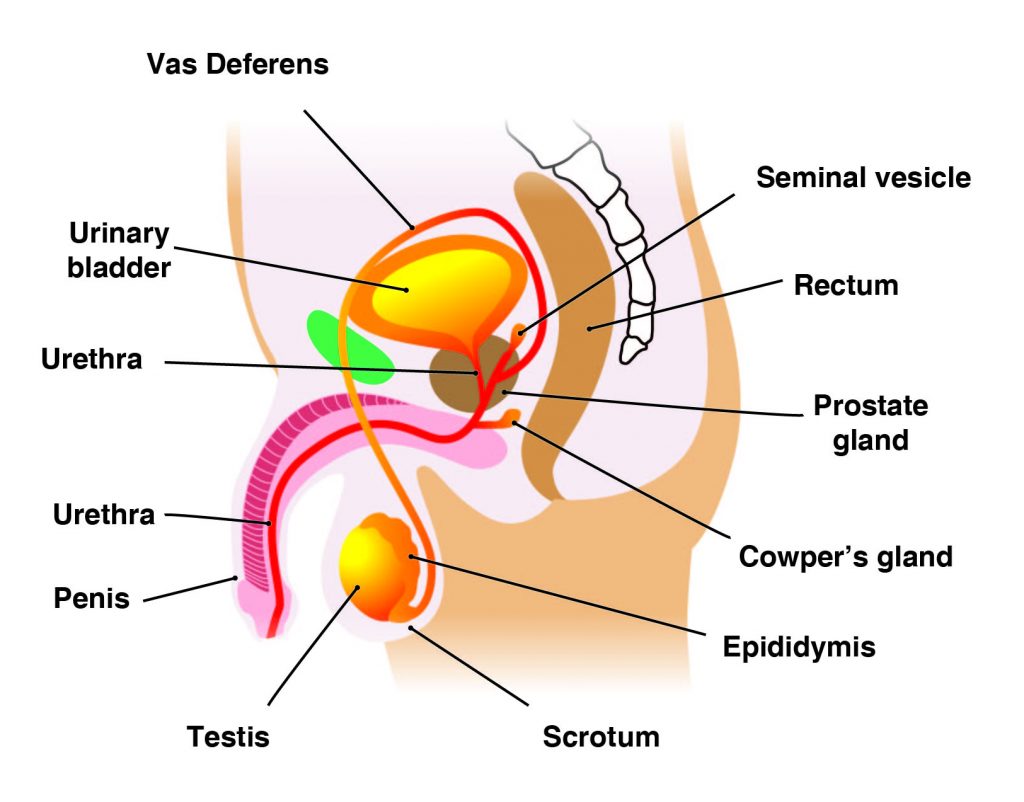
The main male reproductive organs are thepenisand thetesticles, which are located external to the body. The penis is composed of a long shaft and a bulbous end called the glans penis. The glans penis is usually surrounded by an extension of skin called the foreskin.
Thetestes(analogous to the female ovaries), or testicles, are retained in a pouch of skin called thescrotum, which descends from the base of the penis. The scrotum contains nerves and blood vessels needed to support the testicles’ functions. Each testicle (or testis) produces sperm (analogous to the female ova), which are passed into a series of coiled tubules called theepididymis. The epididymis stores and nurtures sperm until they are passed into thevas deferens, a tubule that is about 30 centimeters long, extending from the testicle into the pelvis and ending at the ejaculatory duct.
The epididymis and vas deferens are supported by several accessory glands (the seminal vesicles, the prostate gland, and the Cowper glands) that produce fluid components of semen and support the sperm cells.
Correct Answer is A
Explanation
Robert Hooke discovered the first cells in the mid-eighteenth century. The cell theory is a theory because it is supported by a significant number of experimental findings. The cell theory took many years to be developed because microscopes were not powerful enough to make such observations.
This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is C
Explanation
A pH of 7 is a neutral solution, which is how pure water is classified. Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
Correct Answer is A
Explanation
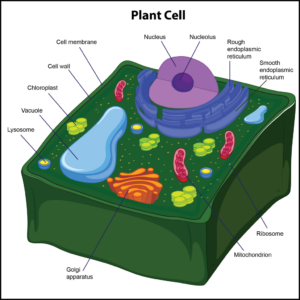
Only plant cells have cell walls, which help protect the cell and provide structural support. The cell wall also enforces the overall structural integrity of the plant cell, and it is found outside the cell membrane. The next organelle is a chloroplast. It is found in the cytoplasm of only plant cells.Chloroplastsare photosynthetic compounds usedto make food for plant cells by harnessing energy from the sun. These organelles play a role in photosynthesis.
Correct Answer is C
Explanation
Inductive reasoning involves making specific observations and using them to make broad statements. The student observes that all of the tigers have the same stripe pattern. He can use this observation to make the broad statement that all the tigers’ offspring will have the same stripe pattern.
Inductive reasoninginvolves drawing a general conclusion from specific observations. This form of reasoning is referred to as the “from the bottom up” approach. Information gathered from specific observations can be used to make a general conclusion about the topic under investigation. In other words, conclusions are based on observed patterns in data.
Correct Answer is A
Explanation
Autotrophs are organisms that use basic raw materials in nature, like the sun, to make energy-rich biomolecules. Minerals are naturally inorganic.
Autotrophsare organisms that make energy-rich biomolecules from raw material in nature. They do this by using basic energy sources such the sun. This explains why most autotrophs rely on photosynthesis to transform sunlight into usable food that can produce energy necessary for life. Plants and certain species of bacteria are autotrophs.
Autotrophs, such as plants, use carbon dioxide (CO2) during photosynthesis to create chemical energy in the form of sugar molecules (like glucose) for growth.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
