In the following single-replacement reaction, ______ replaces ______.
Cl2+2NaI→2NaCl+I2
sodium, iodine
chlorine, iodine
chlorine, sodium
sodium, chlorine
Correct Answer : B
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is D
Explanation
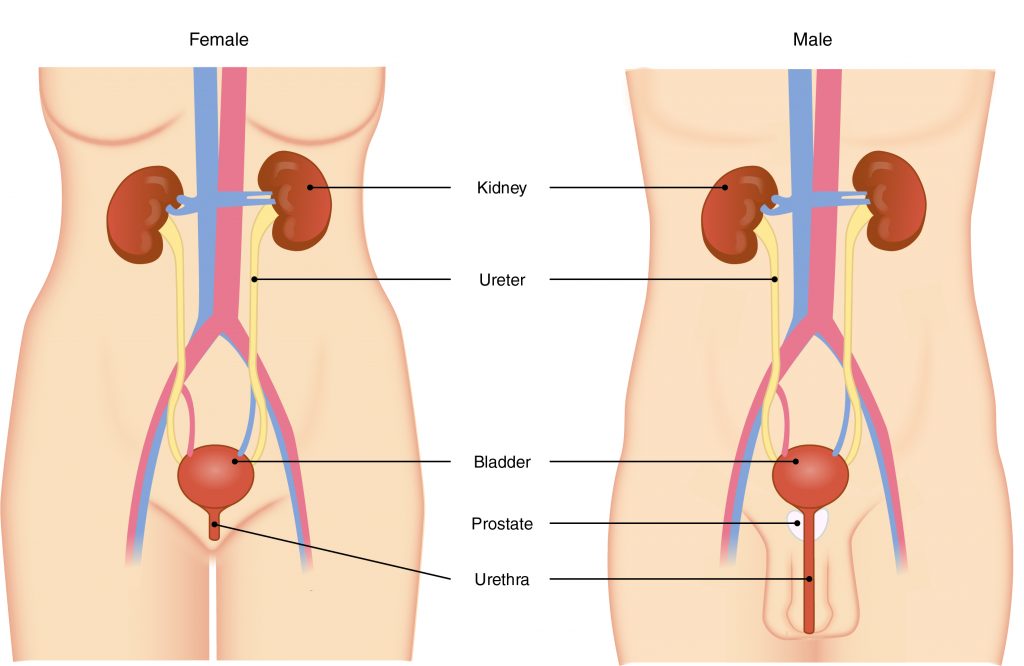
The primary organ of the urinary system is the kidney. Blood from the heart flows through the kidneys via the renal artery. As blood drains from the kidney, it exits through a series of veins, the most prominent of which is the renal vein. When urine is produced, it does not drain through the tubes through which blood flows. Rather, urine flows through two ureters before emptying into the urinary bladder.
The following steps outline how the urinary system works:
- Kidney filters and excretes wastes from blood, producing urine.
- Urine flows down the ureters.
- Urine empties into the bladder and is temporarily stored.
- Bladder, when filled, empties urine out of the body via the urethra.
Correct Answer is D
Explanation
The duodenum is the first part of the small intestines, located between the stomach and the middle part of the small intestines (jejunum). Once food has mixed with acid in the stomach, it moves into the duodenum, where it then mixes with bile from the gallbladder and digestive juices secreted from the pancreas. In the duodenum, absorption of vitamins, minerals, and nutrients begins.
Correct Answer is A
Explanation
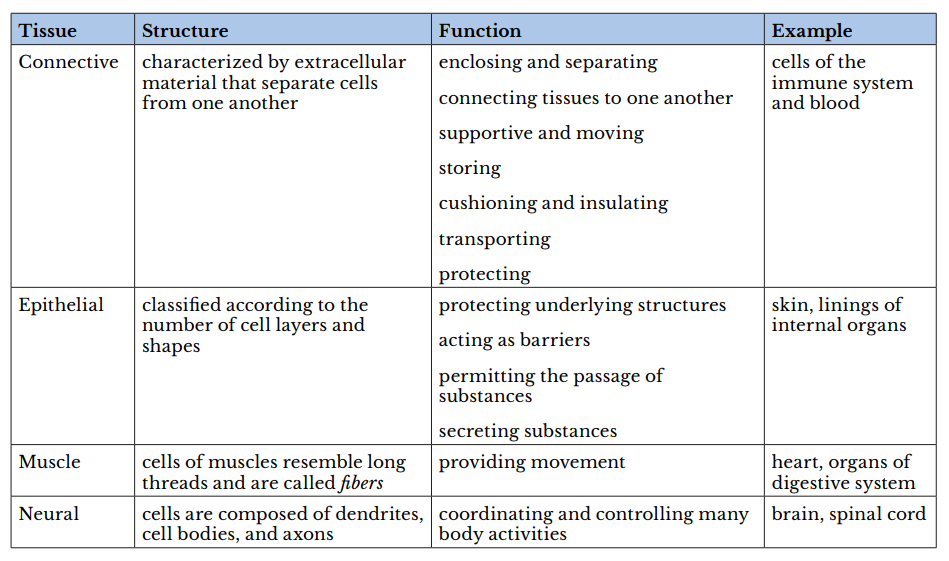
Atissueis a group of cells with similar structure and function and similar extracellular substances located between the cells. The table below describes the four primary tissues found in the human body.
body.
Correct Answer is C
Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
Correct Answer is B
Explanation
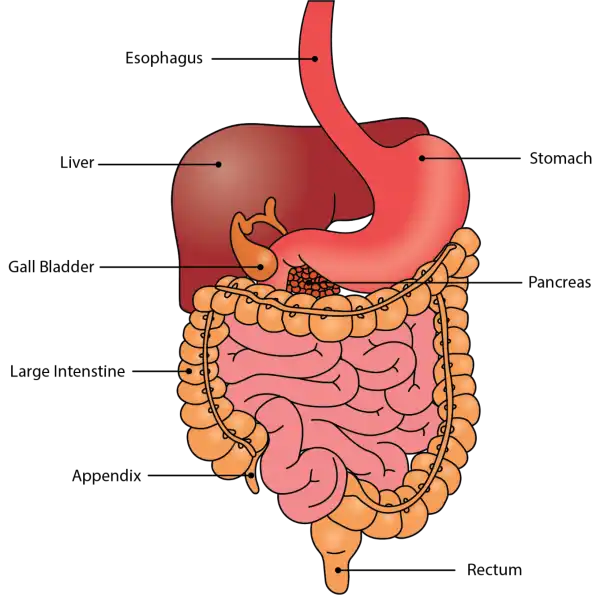
Oral Cavityis the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagusis a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestineis about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colonis about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
Correct Answer is D
Explanation
Mendel developed theories of genetics that scientists around the world use today.
From experiments with garden peas, Mendel developed a simple set of rules that accurately predicted patterns of heredity. He discovered that plants eitherself-pollinateorcross-pollinate, when the pollen from one plant fertilizes the pistil of another plant. He also discovered that traits are eitherdominantorrecessive. Dominant traits are expressed, and recessive traits are hidden.
Mendel’s Theory of Heredity
To explain his results, Mendel proposed a theory that has become the foundation of the science of genetics. The theory has five elements:
- Parents do not transmit traits directly to their offspring. Rather, they pass on units of information calledgenes.
- For each trait, an individual has two factors: one from each parent. If the two factors have the same information, the individual ishomozygousfor that trait. If the two factors are different, the individual isheterozygousfor that trait. Each copy of a factor, orgene, is called anallele.
- The alleles determine the physical appearance, orphenotype. The set of alleles an individual has is itsgenotype.
- An individual receives one allele from each parent.
- The presence of an allele does not guarantee that the trait will be expressed.
Correct Answer is D
Explanation
Human intercourse consists of the male introducing sperm into the female’s reproductive system. Sperm may then pass through the female’s reproductive system to the Fallopian tubes where one sperm fertilizes an ovum, creating azygote. The zygote passes out of the Fallopian tube and implants into the uterine wall to begin gestation. Over nine months, the zygote develops and grows into an embryo and then a fetus. An infant is the baby that is born.
Correct Answer is A
Explanation
Correct Answer is A
Explanation
Autotrophs are organisms that use basic raw materials in nature, like the sun, to make energy-rich biomolecules. Minerals are naturally inorganic.
Autotrophsare organisms that make energy-rich biomolecules from raw material in nature. They do this by using basic energy sources such the sun. This explains why most autotrophs rely on photosynthesis to transform sunlight into usable food that can produce energy necessary for life. Plants and certain species of bacteria are autotrophs.
Autotrophs, such as plants, use carbon dioxide (CO2) during photosynthesis to create chemical energy in the form of sugar molecules (like glucose) for growth.
Correct Answer is B
Explanation
A control group is a factor that does not change during an experiment. Due to this, it is used as a standard for comparison with variables that do change such as a dependent variable.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
