For the reaction below, which of the following would result from increasing the temperature of the environment?
A + B = C + Heat
The concentration of A and B would decrease, and the concentration of C would remain the same.
The reaction would remain at equilibrium, and there would be no change in the concentration of A, B and C.
The concentrations of A, B, and C would all increase.
The concentrations of A and B would increase, and the concentration of C would decrease.
Correct Answer : D
This reaction is exothermic (releases heat), as indicated by the presence of "Heat" on the product side (C + Heat). According to Le Chatelier's Principle, when the temperature of an exothermic reaction is increased, the equilibrium shifts to counteract the added heat by favoring the reverse reaction (where heat is absorbed).
- As a result, the system will shift towards the left (toward the reactants, A and B), to consume the excess heat.
- Therefore, the concentrations of A and B will increase, and the concentration of C will decrease.
The other options do not align with this behavior:
- A. Incorrect, as the concentration of C will change (decrease).
- B. Incorrect, the reaction will shift away from equilibrium due to the temperature change.
- C. Incorrect, the concentration of C will not increase; it will decrease.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
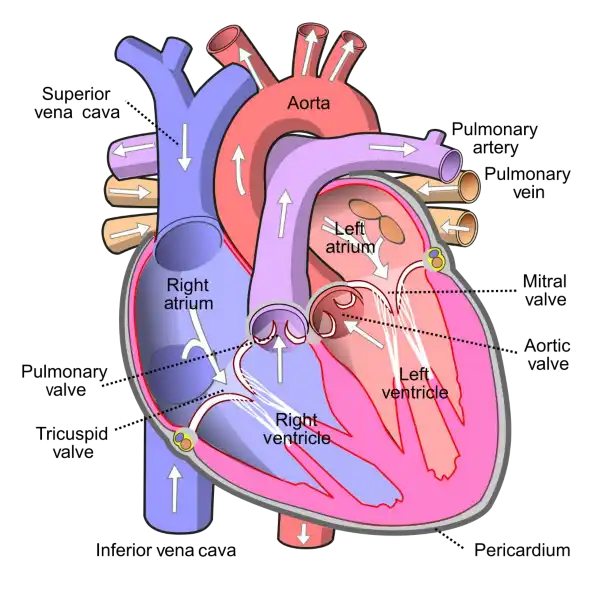
The mitral valve is located between the left atrium and left ventricle of the heart and helps to regulate the flow of blood between these chambers. It consists of two leaflets or flaps that open and close in response to changes in pressure as the heart beats.
During diastole, when the heart is relaxed and filling with blood, the mitral valve opens to allow blood to flow from the left atrium into the left ventricle. During systole, when the heart contracts to pump blood out of the left ventricle and into the systemic circulation, the mitral valve closes to prevent backflow of blood into the left atrium.
The mitral valve is one of four valves in the heart that help to ensure the unidirectional flow of blood through the heart and the rest of the circulatory system. Problems with the mitral valve, such as mitral valve prolapse or mitral stenosis, can lead to a range of symptoms and complications, including shortness of breath, fatigue, chest pain, and heart failure.
 |
Correct Answer is D
Explanation
In the case of methanol poisoning, the metabolism of methanol to formaldehyde is a critical concern because formaldehyde is highly toxic. Ethanol is used as a treatment because it competes with methanol for the same enzyme, methanol oxidase (or alcohol dehydrogenase), effectively inhibiting the metabolism of methanol. By inhibiting the enzyme, ethanol reduces the conversion of methanol to formaldehyde, thereby minimizing its toxic effects.
Here’s why the other options are not suitable treatments:
- A. Methanol oxidase, which would increase the rate of the reaction: This would not be a treatment; it would worsen the situation by promoting the conversion of methanol to toxic formaldehyde.
- B. Methanol, which would saturate the methanol oxidase: This option would also be harmful, as adding more methanol would only lead to more formaldehyde production.
- C. Ice, which would shift the equilibrium of the reaction: The reaction is not a typical equilibrium reaction in this context, and cooling the body does not address the metabolic conversion of methanol to formaldehyde.
Thus, administering ethanol is an effective treatment to prevent the toxic effects of methanol metabolism.
Correct Answer is A
Explanation
The data collected by the researcher on the number of cars passing through a busy intersection at different times of the day for a month would be most useful to analyze traffic paterns during rush hour.
Correct Answer is B
Explanation
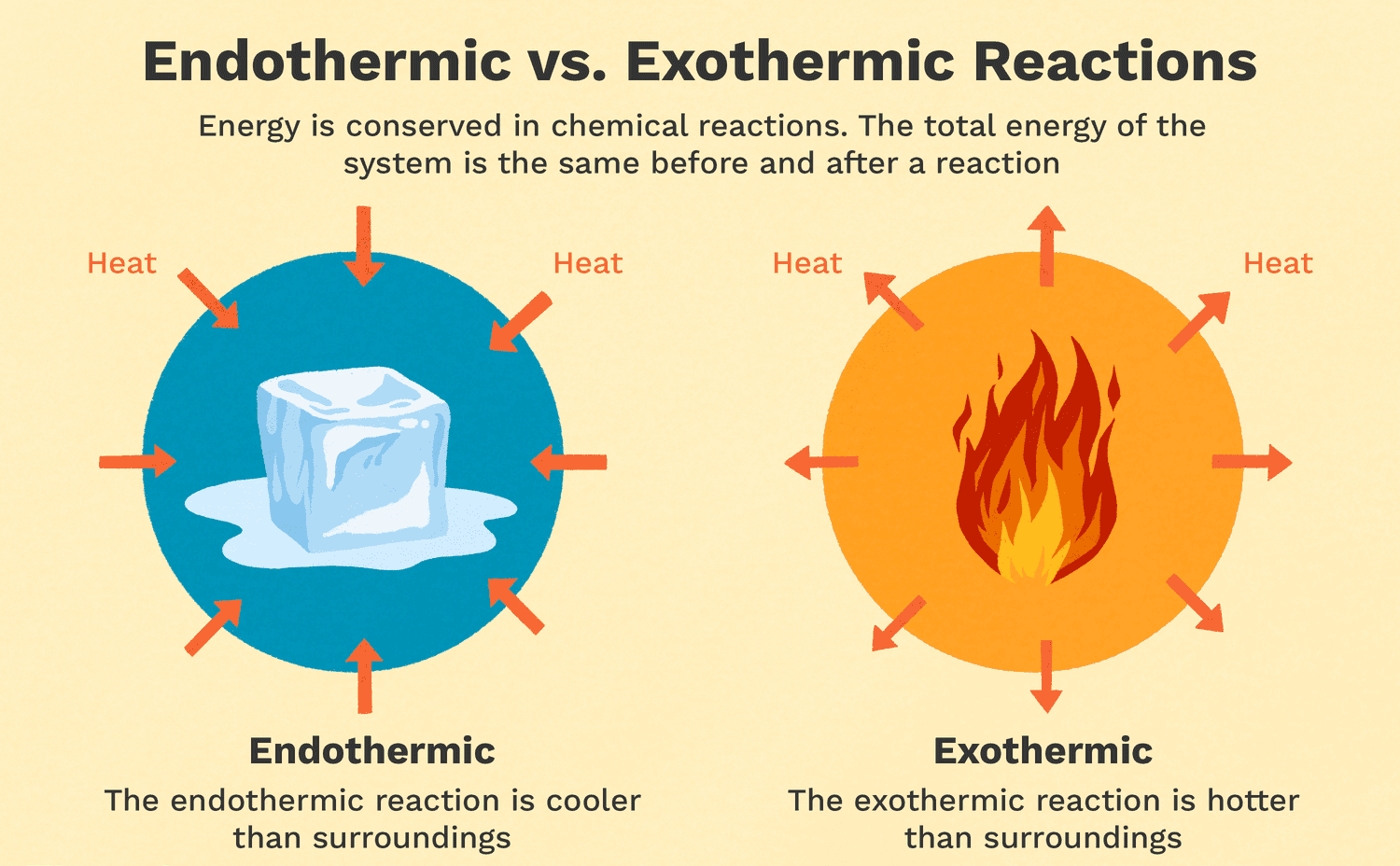
Exothermic reactions are reactions that release energy in the form of heat, light, or sound. Burning wood is an example of an exothermic reaction because it releases heat and light. As the wood reacts with oxygen in the air, it undergoes a combustion reaction that releases energy in the form of heat and light. Melting ice is an endothermic reaction because it requires energy input to melt the solid ice into liquid water. Cooking an egg is a chemical reaction that involves denaturing the proteins in the egg, but it is not necessarily exothermic or endothermic. Dissolving sugar in water is also not an example of an exothermic reaction because it does not release energy in the form of heat, light, or sound.
Correct Answer is D
Explanation
This reaction is exothermic (releases heat), as indicated by the presence of "Heat" on the product side (C + Heat). According to Le Chatelier's Principle, when the temperature of an exothermic reaction is increased, the equilibrium shifts to counteract the added heat by favoring the reverse reaction (where heat is absorbed).
- As a result, the system will shift towards the left (toward the reactants, A and B), to consume the excess heat.
- Therefore, the concentrations of A and B will increase, and the concentration of C will decrease.
The other options do not align with this behavior:
- A. Incorrect, as the concentration of C will change (decrease).
- B. Incorrect, the reaction will shift away from equilibrium due to the temperature change.
- C. Incorrect, the concentration of C will not increase; it will decrease.
Correct Answer is D
Explanation
 |
Correct Answer is B
Explanation
One of the key differences between skeletal muscles and cardiac muscles is the presence of intercalated discs in cardiac muscle tissue. These discs are specialized structures that facilitate communication and synchronization between cardiac muscle cells, allowing the heart to contract as a unified organ.
The other options are incorrect:
- A. Skeletal muscles are autorhythmic, whereas cardiac muscles are not: This is incorrect because cardiac muscles are autorhythmic; they can generate their own rhythmic contractions. Skeletal muscles require nervous system stimulation to contract.
- C. Skeletal muscles are found in the viscera, whereas cardiac muscles are found in the cranium: This is incorrect; skeletal muscles are primarily associated with the skeleton (attached to bones) and are not typically found in the viscera, while cardiac muscle is found in the heart.
- D. Cardiac muscles are voluntary, whereas skeletal muscles are involuntary: This is incorrect; skeletal muscles are voluntary (under conscious control), while cardiac muscles are involuntary (not under conscious control).
Therefore, the correct distinction is that cardiac muscles contain intercalated discs, while skeletal muscles do not.
Correct Answer is C
Explanation
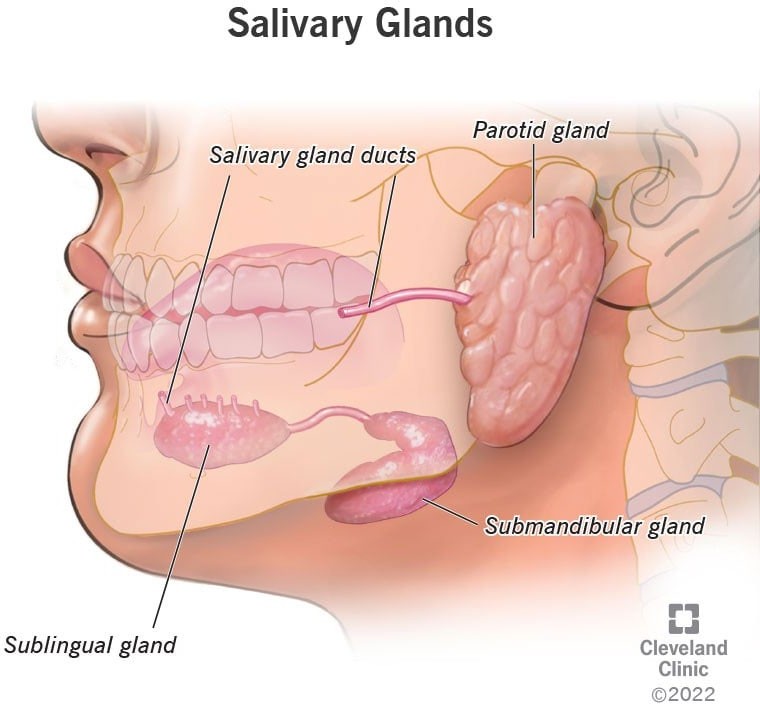
The three major pairs of salivary glands are the parotid glands, sublingual glands, and submandibular glands.
- Parotid glands are located just in front of your ears.
- Sublingual glands are located below either side of your tongue, under the floor of your mouth.
- Submandibular glands are located below your jaw.
 |
Correct Answer is A
Explanation
The diaphragm is a dome-shaped muscle that plays a key role in breathing. It separates the thoracic cavity, which contains the heart and lungs, from the abdominal cavity. When the diaphragm contracts, it moves downward and increases the volume of the thoracic cavity, allowing air to flow into the lungs. When it relaxes, it moves upward and decreases the volume of the thoracic cavity, forcing air out of the lungs.
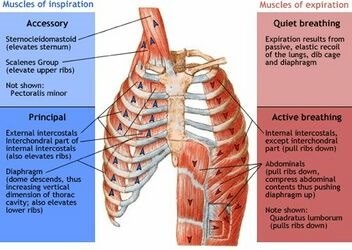 |
Correct Answer is A
Explanation
The chemical formula for water is H2O. It consists of two hydrogen atoms and one oxygen atom.
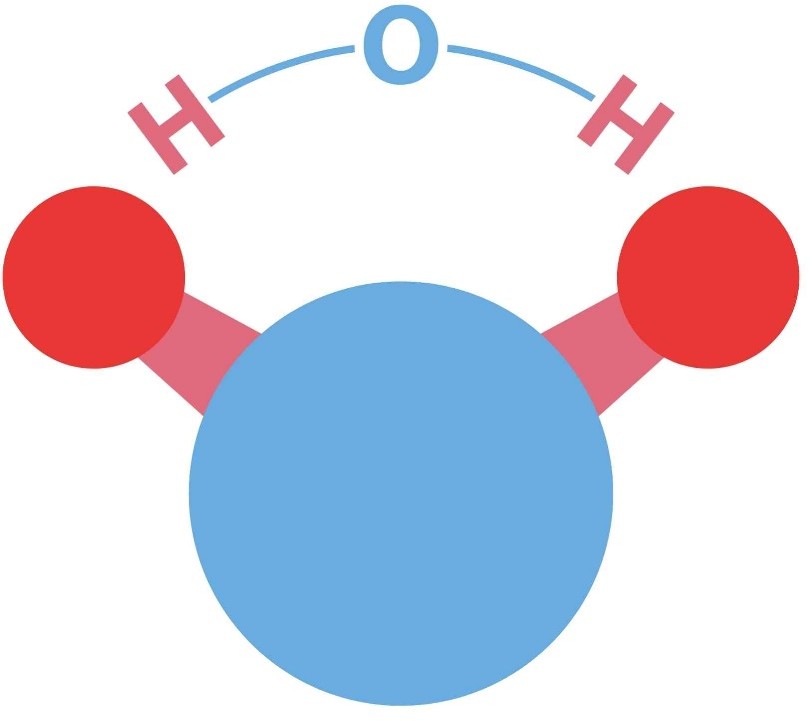 |
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
