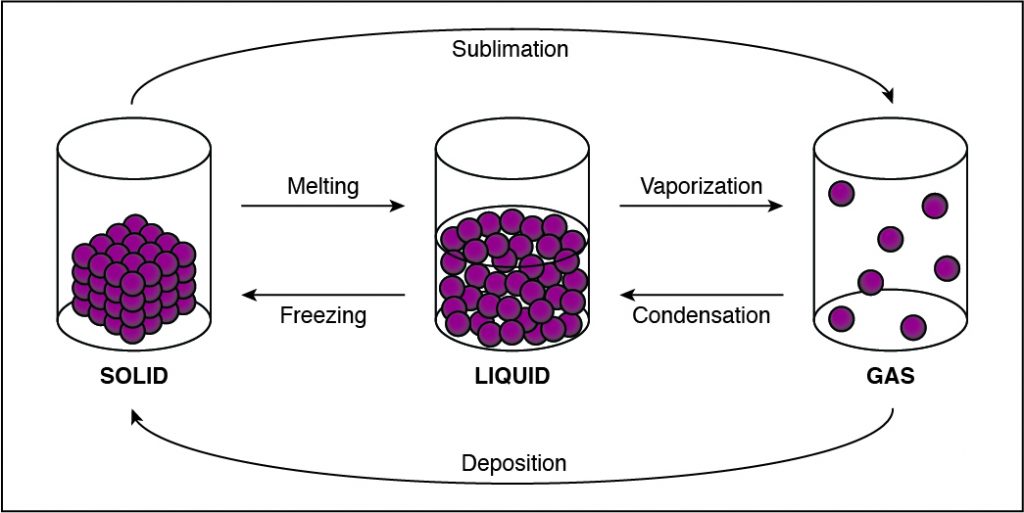
During which of the following phase changes will the cohesion between the particles in a substance decrease?
Condensation
Deposition
Freezing
Vaporization
Correct Answer : D
If the cohesion between particles decreases, then the particles must be undergoing a phase change that allows particles to move farther apart. This happens when a substance vaporizes and turns from liquid to gas. Any phase change that moves to the right in the diagram above requires energy to be added to the system because the substance has more energy at the end of the phase change. The phase changes are melting, vaporization (boiling), and sublimation. When energy is added, particles move faster and can break away from each other more easily as they move to a state of matter with a higher amount of energy. This is most commonly done by heating the substance.
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is B
Explanation
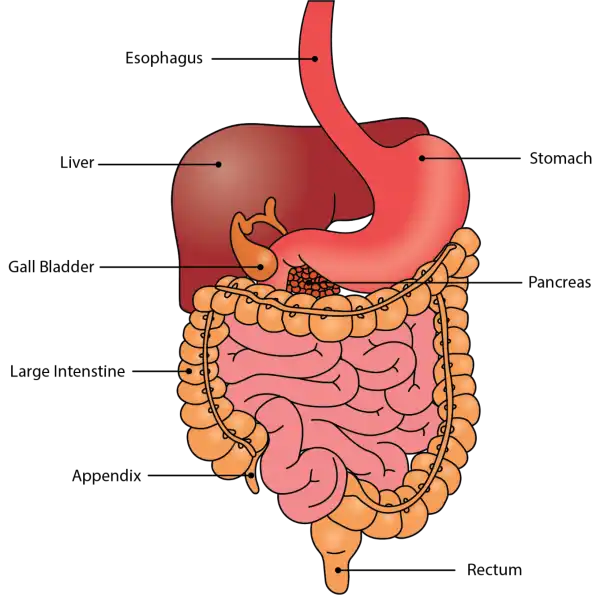
Oral Cavityis the first part of the digestive system. It is bounded by the lips and cheeks and contains the teeth and tongue. Its primary function is to masticate, or chew, and moisten the food.
Pharynx, or throat, connects the mouth to the esophagus.
Esophagusis a muscular tube about 25 centimeters long. Food travels down it to the cardiac sphincter of the stomach.
Pyloric sphincter. The exit of the stomach.
Small intestineis about 6 meters long and consists of three parts: duodenum, jejunum, and ileum.
Large intestine, consists of the cecum, colon, rectum, and anal canal. The cecum is located where the small and large intestine meet. The primary function of the large intestine is to compress the waste and collect any excess water that can be recycled.
Colonis about 1.5 to 1.8 meters long and consists of four parts: the ascending, transverse, descending, and sigmoid colon.
Correct Answer is B
Explanation
There are two major types of receptor molecules that respond to an intercellular chemical signal:
- Intracellular receptors: These receptors are located in either the cytoplasm or the nucleus of the cell. Signals diffuse across the cell membrane and bind to the receptor sites on intracellular receptors, of the same cell.
- Membrane-bound receptors: These receptors extend across the cell membrane, with their receptor sites on the outer surface of the cell membrane. They respond to intercellular chemical signals that are large, water-soluble molecules that do not diffuse across the cell membrane.
Correct Answer is A
Explanation
Robert Hooke discovered the first cells in the mid-eighteenth century. The cell theory is a theory because it is supported by a significant number of experimental findings. The cell theory took many years to be developed because microscopes were not powerful enough to make such observations.
This theory, or in-depth explanation, about cells consists of three parts:
- All living things are composed of one or more cells.
- Cells are alive and represent the basic unit of life.
- All cells are produced from pre-existing cells.
Correct Answer is D
Explanation
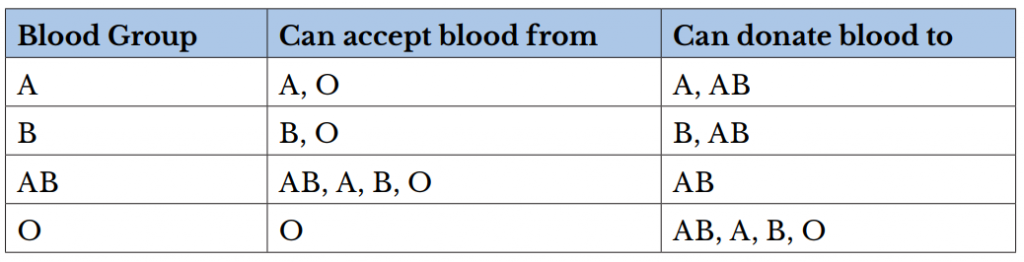
A person can be a universal blood donor or acceptor. A universal blood donor has type O blood, while a universal blood acceptor has type AB blood.
There are several different types or groups of blood, andthe major groups are A, B, AB, and O. Blood group is a way to classify blood according to inherited differences of red blood cellantigensfound on the surface of a red blood cell. The type ofantibodyin blood also identifies a particular blood group. Antibodies are proteins found in the plasma. They function as part of the body’s natural defense to recognize foreign substances and alert the immune system.
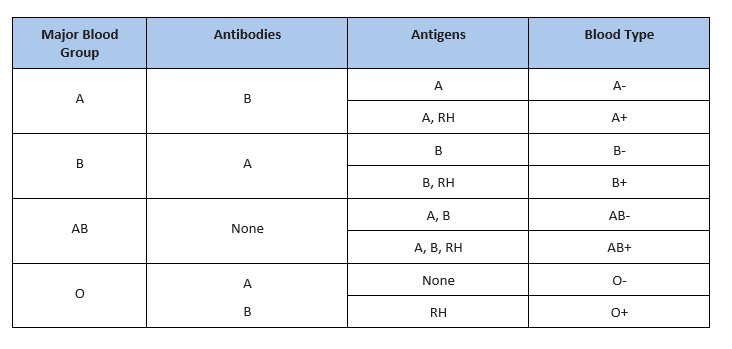
Depending on which antigen is inherited, parental offspring will have one of the four major blood groups. Collectively, the following major blood groups comprise the ABO system:
- Blood group A: Displays type A antigens on the surface of a red blood cell and contains B antibodies in the plasma.
- Blood group B: Displays type B antigens on the red blood cell’s surface and contains A antibodies in the plasma.
- Blood group O: Does not display A or B antigens on the surface of a red blood cell. Both A and B antibodies are in the plasma.
- Blood group AB: Displays type A and B antigens on the red blood cell’s surface, but neither A nor B antibodies are in the plasma
In addition to antigens, the Rh factor protein may exist on a red blood cell’s surface. Because this protein can be either present (+) or absent (-), it increases the number of major blood groups from four to eight: A+, A-, B+, B-, O+, O-, AB+, and AB-.
Correct Answer is B
Explanation
A control group is a factor that does not change during an experiment. Due to this, it is used as a standard for comparison with variables that do change such as a dependent variable.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
Correct Answer is D
Explanation
Because it has more protons than electrons, this atom has a positive charge and can be classified as a cation. When a metal such as sodium reacts to become stable, it loses its valence electrons. At first, it is a neutral atom with 11 protons and 11 electrons. When it loses an electron, the number of protons does not change, and the atom has 11 protons and 10 electrons. Because there is one more positively charged proton, acationforms. A cation is an ion with a net positive charge.
Correct Answer is C
Explanation
The particles in a sample of gas are farther apart than in solids or liquids and therefore have the lowest amount of cohesion.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
Correct Answer is A
Explanation
The main male reproductive organs are thepenisand thetesticles, which are located external to the body. The penis is composed of a long shaft and a bulbous end called the glans penis. The glans penis is usually surrounded by an extension of skin called the foreskin.
Thetestes(analogous to the female ovaries), or testicles, are retained in a pouch of skin called thescrotum, which descends from the base of the penis. The scrotum contains nerves and blood vessels needed to support the testicles’ functions. Each testicle (or testis) produces sperm (analogous to the female ova), which are passed into a series of coiled tubules called theepididymis. The epididymis stores and nurtures sperm until they are passed into thevas deferens, a tubule that is about 30 centimeters long, extending from the testicle into the pelvis and ending at the ejaculatory duct.
The epididymis and vas deferens are supported by several accessory glands (the seminal vesicles, the prostate gland, and the Cowper glands) that produce fluid components of semen and support the sperm cells.
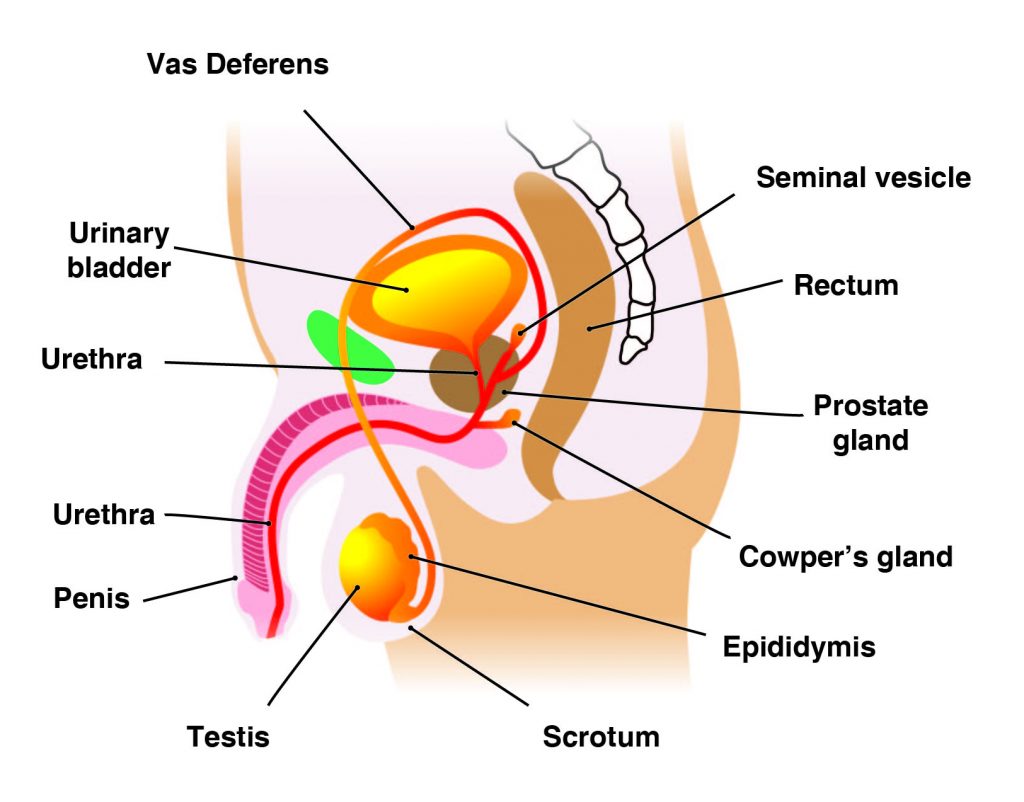
Correct Answer is C
Explanation
One step of the scientific method is to analyze information or data collected from the experiment to conclude whether the hypothesis is supported.
Recall that these make up thescientific method,described below:
- Problem:The question created because of an observation.Example: Does the size of a plastic object affect how fast it naturally degrades in a lake?
- Research:Reliable information available about what is observed.Example: Learn how plastics are made and understand the properties of a lake.
- Hypothesis:A predicted solution to the question or problem.Example: If the plastic material is small, then it will degrade faster than a large particle.
- Experiment:A series of tests used to evaluate the hypothesis. Experiments consist of anindependent variablethat the researcher modifies and adependent variablethat changes due to the independent variable. They also include acontrol groupused as a standard to make comparisons.
- Example: Collect plastic particles both onshore and offshore of the lake over time. Determine the size of the particles and describe the lake conditions during this time period.
- Observe:Analyze data collected during an experiment to observe patterns.
- Example: Analyze the differences between the numbers of particles collected in terms of size.
- Conclusion:State whether the hypothesis is rejected or accepted and summarize all results.
- Communicate:Report findings so others can replicate and verify the results.
Correct Answer is D
Explanation
The duodenum is the first part of the small intestines, located between the stomach and the middle part of the small intestines (jejunum). Once food has mixed with acid in the stomach, it moves into the duodenum, where it then mixes with bile from the gallbladder and digestive juices secreted from the pancreas. In the duodenum, absorption of vitamins, minerals, and nutrients begins.
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
