Practice Question
A solution that is at a pH of 1 ______ than a solution with a pH of 9.
has fewer hydrogen ions
has more hydrogen ions
has an equal number of hydrogen ions
is less concentrated
Correct Answer : B
TEAS 7 Exam Quiz Bank
HESI A2 Exam Quiz Bank
Find More Questions 📚
Teas 7 Questions: We got the latest updated TEAS 7 questions
100% Money Refund: 100% money back guarantee if you take our full
assessment pass with 80% and fail the actual exam.
Live Tutoring: Fully customized live tutoring lessons.
Guaranteed A Grade: All students who use our services pass with 90%
guarantee.
Related Questions
Correct Answer is C
Explanation
The pH scale is a quantitative measure of the concentration of hydrogen ions in a solution. It tuns from 1 to 14 and the pH values less than 7 signify a solution is acidic, while those values greater than 7 are basic or alkaline. A solution with a pH of 7 is neutral. The pH scale is logarithmic and every unit of pH corresponds tie 10-fold change in acidity. As the pH decreases, the acidity of the solution increases. Therefore, a solution with a pH of 3 is 10 times more acidic than a solution with a pH of 4.
Correct Answer is D
Explanation
Th pH scale runs from 0 to 14. Acid substances have pH values between 0 and 7 While alkaline solutions have pH values between 7 and 14. A neutral solution has a pH value of 7. Thus, from the question above, a pH value of 12 represent an alkaline solution.
Correct Answer is B
Explanation
The physiological function of the body depends on the balance between the concentrations of acids and bases in the blood. For a better body function, the blood pH falls in the range of 7.45 and 7.35. beyond this point, the blood my become too acidic or basic and may have drastic effect on the physiologic function of the body. To maintain this blood at constant, the buffer system comes into play. It does not allow the pH to drop or increase drastically. From the properties of acids and bases, acids donate protons while bases are proton acceptors. In our bodies, there are three buffer systems:
- Carbonate/carbonic acid buffer
- Phosphate buffer
- Plasma proteins
Th carbonate/carbonic acid buffer plays a key role in acid-base balance in our bodies. This buffer system is regulated by the respiratory system. If there is less H ions on the system, the carbonic acid dissociates and donate H ions as show in the reaction below, which thus declines the pH of the blood.
H2CO3 + H2O ?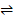 H3O+ + HCO3-
H3O+ + HCO3-
Correct Answer is B
No explanation
This question was extracted from the actual TEAS Exam. Ace your TEAS exam with the actual TEAS 7 questions, Start your journey with us today
Visit Naxlex, the Most Trusted TEAS TEST Platform With Guaranteed Pass of 90%.
Money back guarantee if you use our service and fail the actual exam. Option of personalised live tutor on your area of weakness.
